当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Diels–Alder Reactions of Tethered Enoate Substituted Furans Induced by Dialkylaluminum Chloride
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.joc.7b02117 Sibylle Riedel 1 , Cäcilia Maichle-Mössmer 2 , Martin E. Maier 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.joc.7b02117 Sibylle Riedel 1 , Cäcilia Maichle-Mössmer 2 , Martin E. Maier 1
Affiliation
|
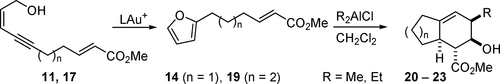
Gold(I)-catalyzed cycloisomerization of enynols 11 and 17, obtained by Sonogashira coupling, led to the tethered enoate-substituted furans 14 and 19. While attempts at thermal and several Lewis acid induced intramolecular Diels–Alder reactions remained fruitless, dialkylaluminum chloride led to the formation of hexahydroindene and octahydronaphthalene derivatives 20–23. Their formation can be explained by Lewis acid induced opening of the epoxy bridge with transfer of one alkyl group to the intermediate cycloadduct.
中文翻译:

氯化二烷基铝诱导的束缚内酯取代呋喃的分子内Diels-Alder反应
通过Sonogashira偶联获得的金(I)催化的烯醇11和17的环异构化,导致束缚的烯酸酯取代的呋喃14和19。而在热和几个路易斯酸尝试诱导分子内狄尔斯-阿尔德反应仍然不结果,二烷基氯化铝导致hexahydroindene和八氢萘衍生物的形成20 - 23。它们的形成可以通过路易斯酸诱导的环氧桥的打开以及一个烷基转移至中间环加合物来解释。
更新日期:2017-11-16
中文翻译:

氯化二烷基铝诱导的束缚内酯取代呋喃的分子内Diels-Alder反应
通过Sonogashira偶联获得的金(I)催化的烯醇11和17的环异构化,导致束缚的烯酸酯取代的呋喃14和19。而在热和几个路易斯酸尝试诱导分子内狄尔斯-阿尔德反应仍然不结果,二烷基氯化铝导致hexahydroindene和八氢萘衍生物的形成20 - 23。它们的形成可以通过路易斯酸诱导的环氧桥的打开以及一个烷基转移至中间环加合物来解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号