当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Short Enantioselective Total Syntheses of Cheloviolenes A and B and Dendrillolide C via Convergent Fragment Coupling Using a Tertiary Carbon Radical
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.joc.7b02458 Michelle R. Garnsey 1 , Yuriy Slutskyy 1 , Christopher R. Jamison 1 , Peng Zhao 1 , Juyeol Lee 1 , Young Ho Rhee 2 , Larry E. Overman 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.joc.7b02458 Michelle R. Garnsey 1 , Yuriy Slutskyy 1 , Christopher R. Jamison 1 , Peng Zhao 1 , Juyeol Lee 1 , Young Ho Rhee 2 , Larry E. Overman 1
Affiliation
|
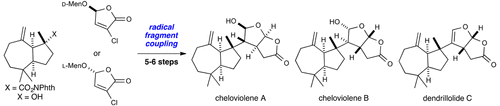
The development of a convergent fragment coupling strategy for the enantioselective total syntheses of a group of rearranged spongian diterpenoids that harbor the cis-2,8-dioxabicyclo[3.3.0]octan-3-one unit is described. The key bond disconnection relies on a late-stage fragment coupling between a tertiary carbon radical and an electron-deficient alkene to unite two ring systems and form two new stereocenters, one of which is quaternary, in a stereoselective and efficient manner. This strategy is applied toward scalable 14–15 step syntheses of three rearranged spongian diterpenoids, cheloviolenes A and B, and dendrillolide C.
中文翻译:

通过使用第三级碳自由基的会聚片段偶联,对螯合物A和B以及树枝状内酯C进行短对映选择性全合成
描述了收敛性片段偶联策略的发展,该策略用于对具有顺式-2,8-二氧杂双环[3.3.0] octan-3-one单元的一组重排海绵状二萜进行对映选择性全合成。关键键的断开依赖于叔碳基团和缺电子烯烃之间的后期片段偶联,以结合两个环系统并以立体选择性和有效方式形成两个新的立体中心,其中一个是四元的。此策略适用于三个可重排的海绵状二萜,鹅绒素A和B以及树突内酯C的可扩展的14-15步合成。
更新日期:2017-11-13
中文翻译:

通过使用第三级碳自由基的会聚片段偶联,对螯合物A和B以及树枝状内酯C进行短对映选择性全合成
描述了收敛性片段偶联策略的发展,该策略用于对具有顺式-2,8-二氧杂双环[3.3.0] octan-3-one单元的一组重排海绵状二萜进行对映选择性全合成。关键键的断开依赖于叔碳基团和缺电子烯烃之间的后期片段偶联,以结合两个环系统并以立体选择性和有效方式形成两个新的立体中心,其中一个是四元的。此策略适用于三个可重排的海绵状二萜,鹅绒素A和B以及树突内酯C的可扩展的14-15步合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号