当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Non-Metal-Catalyzed Heterodehydrocoupling of Phosphines and Hydrosilanes: Mechanistic Studies of B(C6F5)3-Mediated Formation of P–Si Bonds
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/jacs.7b09175 Lipeng Wu 1 , Saurabh S. Chitnis 1 , Haijun Jiao 2 , Vincent T. Annibale 1 , Ian Manners 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/jacs.7b09175 Lipeng Wu 1 , Saurabh S. Chitnis 1 , Haijun Jiao 2 , Vincent T. Annibale 1 , Ian Manners 1
Affiliation
|
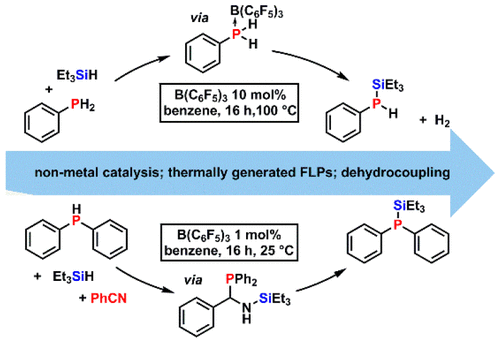
Non-metal-catalyzed heterodehydrocoupling of primary and secondary phosphines (R1R2PH, R2 = H or R1) with hydrosilanes (R3R4R5SiH, R4, R5 = H or R3) to produce synthetically useful silylphosphines (R1R2P–SiR3R4R5) has been achieved using B(C6F5)3 as the catalyst (10 mol %, 100 °C). Kinetic studies demonstrated that the reaction is first-order in hydrosilane and B(C6F5)3 but zero-order in phosphine. Control experiments, DFT calculations, and DOSY NMR studies suggest that a R1R2HP·B(C6F5)3 adduct is initially formed and undergoes partial dissociation to form an “encounter complex”. The latter mediates frustrated Lewis pair type Si–H bond activation of the silane substrates. We also found that B(C6F5)3 catalyzes the homodehydrocoupling of primary phosphines to form cyclic phosphine rings and the first example of a non-metal-catalyzed hydrosilylation of P–P bonds to produce silylphosphines (R1R2P–SiR3R4R5). Moreover, the introduction of PhCN to the reactions involving secondary phosphines with hydrosilanes allowed the heterodehydrocoupling reaction to proceed efficiently under much milder conditions (1.0 mol % B(C6F5)3 at 25 °C). Mechanistic studies, as well as DFT calculations, revealed that PhCN plays a key mechanistic role in facilitating the dehydrocoupling reactions rather than simply functioning as H2-acceptor.
中文翻译:

膦和氢硅烷的非金属催化杂多氢偶联:B(C 6 F 5)3介导的P-Si键形成的机理研究
伯膦和仲膦(R 1 R 2 PH,R 2 = H或R 1)与氢硅烷(R 3 R 4 R 5 SiH,R 4,R 5 = H或R 3)的非金属催化杂脱氢偶联反应合成上有用的甲硅烷基膦(R 1 R 2 P–SiR 3 R 4 R 5)已经使用B(C 6 F 5)3作为催化剂(10 mol%,100°C)获得。动力学研究表明,该反应在氢硅烷和B(C6 F 5)3但在膦中为零级。对照实验,DFT计算和DOSY NMR研究表明,最初形成R 1 R 2 HP·B(C 6 F 5)3加合物,并进行部分离解以形成“相遇复合物”。后者介导了硅烷基材的沮丧的路易斯对型Si–H键活化。我们还发现,B(C 6 F 5)3催化伯膦的均氢脱氢偶联形成环状膦环,并且是非金属催化的P–P键氢化硅烷化生成甲硅烷基膦的第一个例子(R 1 R2 P–SiR 3 R 4 R 5)。而且,将PhCN引入涉及仲膦与氢硅烷的反应中使得杂脱氢偶联反应在更温和的条件下(在25℃下为1.0mol%B(C 6 F 5)3)有效地进行。机理研究以及DFT计算表明,PhCN在促进脱氢偶联反应中起着关键的机理作用,而不是简单地充当H 2-受体。
更新日期:2017-11-16
中文翻译:

膦和氢硅烷的非金属催化杂多氢偶联:B(C 6 F 5)3介导的P-Si键形成的机理研究
伯膦和仲膦(R 1 R 2 PH,R 2 = H或R 1)与氢硅烷(R 3 R 4 R 5 SiH,R 4,R 5 = H或R 3)的非金属催化杂脱氢偶联反应合成上有用的甲硅烷基膦(R 1 R 2 P–SiR 3 R 4 R 5)已经使用B(C 6 F 5)3作为催化剂(10 mol%,100°C)获得。动力学研究表明,该反应在氢硅烷和B(C6 F 5)3但在膦中为零级。对照实验,DFT计算和DOSY NMR研究表明,最初形成R 1 R 2 HP·B(C 6 F 5)3加合物,并进行部分离解以形成“相遇复合物”。后者介导了硅烷基材的沮丧的路易斯对型Si–H键活化。我们还发现,B(C 6 F 5)3催化伯膦的均氢脱氢偶联形成环状膦环,并且是非金属催化的P–P键氢化硅烷化生成甲硅烷基膦的第一个例子(R 1 R2 P–SiR 3 R 4 R 5)。而且,将PhCN引入涉及仲膦与氢硅烷的反应中使得杂脱氢偶联反应在更温和的条件下(在25℃下为1.0mol%B(C 6 F 5)3)有效地进行。机理研究以及DFT计算表明,PhCN在促进脱氢偶联反应中起着关键的机理作用,而不是简单地充当H 2-受体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号