当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Palladium-Catalyzed Intramolecular α-Arylative Desymmetrization of 1,3-Diketones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/jacs.7b10365 Chendan Zhu 1 , Dingyi Wang 1 , Yue Zhao 1 , Wei-Yin Sun 1 , Zhuangzhi Shi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/jacs.7b10365 Chendan Zhu 1 , Dingyi Wang 1 , Yue Zhao 1 , Wei-Yin Sun 1 , Zhuangzhi Shi 1
Affiliation
|
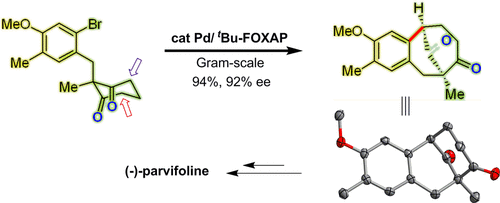
An efficient enantioselective protocol has been reported to build highly oxygenated and densely substituted bicyclo[m.n.1] skeletons through intramolecular asymmetric α-arylative desymmetrization of 1,3-diketones. Employing Pd catalyst and FOXAP ligand, various bicyclo[m.n.1] skeleton with different size can be accessed with high enantio- and diastereoselectivities. Utilizing the present method as a key step, formal asymmetric total synthesis of the (−)-parvifoline has been demonstrated.
中文翻译:

1,3-二酮的对映选择性钯催化的分子内α-芳基不对称化
据报道,一种有效的对映选择性方案可构建高度氧化和密集取代的双环[ m]。Ñ 0.1]到的1,3-二酮的分子内不对称α-arylative desymmetrization骨架。使用Pd催化剂和FOXAP配体,各种双环[ m。[ 0.1] [n.1]骨架可以通过高对映选择性和非对映选择性获得。利用本发明的方法作为关键步骤,已经证明了(-)-细小佛碱的形式不对称全合成。
更新日期:2017-11-16
中文翻译:

1,3-二酮的对映选择性钯催化的分子内α-芳基不对称化
据报道,一种有效的对映选择性方案可构建高度氧化和密集取代的双环[ m]。Ñ 0.1]到的1,3-二酮的分子内不对称α-arylative desymmetrization骨架。使用Pd催化剂和FOXAP配体,各种双环[ m。[ 0.1] [n.1]骨架可以通过高对映选择性和非对映选择性获得。利用本发明的方法作为关键步骤,已经证明了(-)-细小佛碱的形式不对称全合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号