当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Theoretical Studies on Iron-Promoted Oxidative Annulation of Arylglyoxal with Alkyne: Unusual Addition and Migration on the Aryl Ring
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/jacs.7b05981 Chen-Hsun Hung,Parthasarathy Gandeepan,Lin-Chieh Cheng,Liang-Yu Chen,Mu-Jeng Cheng,Chien-Hong Cheng
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/jacs.7b05981 Chen-Hsun Hung,Parthasarathy Gandeepan,Lin-Chieh Cheng,Liang-Yu Chen,Mu-Jeng Cheng,Chien-Hong Cheng
|
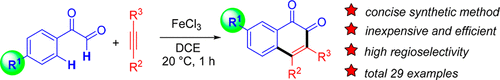
An Fe(III)-promoted oxidative annulation reaction was developed for the synthesis of 1,2-naphthoquinones. A variety of substituted arylglyoxals and internal alkynes undergo the transformation in the presence of FeCl3 at room temperature to afford the 1,2-naphthoquinone products in good yields in a short reaction time. Interestingly, the products show unusual pseudomigration of the substituent on the arene ring of arylglyoxals. A possible mechanism involving Fe(III)-promoted formation of a vinyl cation from arylglyoxal and alkyne, electrophilic addition of the vinyl cation to the ipso carbon of the aryl group to give a spiral intermediate, and then migration of the keto carbon to the ortho carbon was proposed as key steps and verified using quantum mechanics.
中文翻译:

铁对炔乙醛与炔烃的氧化环化反应的实验和理论研究:芳基环上异常的加成和迁移
开发了Fe(III)促进的氧化环化反应,用于合成1,2-萘醌。在室温下,在FeCl 3存在下,各种取代的芳基乙二醛和内部炔烃进行转化,从而在较短的反应时间内以良好的收率得到1,2-萘醌产物。有趣的是,产物显示出芳基乙二醛的芳族环上的取代基的异常假迁移。可能的机制包括Fe(III)促进由芳基乙二醛和炔烃形成乙烯基阳离子,将乙烯基阳离子亲电加成到芳基的ipso碳上以形成螺旋中间体,然后将酮碳迁移到邻位碳被认为是关键步骤,并使用量子力学进行了验证。
更新日期:2017-11-16
中文翻译:

铁对炔乙醛与炔烃的氧化环化反应的实验和理论研究:芳基环上异常的加成和迁移
开发了Fe(III)促进的氧化环化反应,用于合成1,2-萘醌。在室温下,在FeCl 3存在下,各种取代的芳基乙二醛和内部炔烃进行转化,从而在较短的反应时间内以良好的收率得到1,2-萘醌产物。有趣的是,产物显示出芳基乙二醛的芳族环上的取代基的异常假迁移。可能的机制包括Fe(III)促进由芳基乙二醛和炔烃形成乙烯基阳离子,将乙烯基阳离子亲电加成到芳基的ipso碳上以形成螺旋中间体,然后将酮碳迁移到邻位碳被认为是关键步骤,并使用量子力学进行了验证。











































 京公网安备 11010802027423号
京公网安备 11010802027423号