当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereodivergent Asymmetric Carboamination/Annulation of Cyclopropenes with Aminoalkenes by Chiral Lanthanum Catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/jacs.7b10786 Huai-Long Teng 1 , Yong Luo 2 , Masayoshi Nishiura 1, 2 , Zhaomin Hou 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/jacs.7b10786 Huai-Long Teng 1 , Yong Luo 2 , Masayoshi Nishiura 1, 2 , Zhaomin Hou 1, 2
Affiliation
|
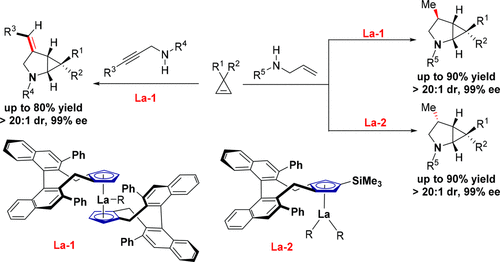
Stereodivergent asymmetric catalysis is an important technology that can allow efficient access to various stereoisomers of a given product with multiple stereocenters from the same set of starting materials, but its application to the synthesis of a highly strained cyclopropane compound has remained unexplored to date. We report here the first diastereodivergent enantioselective synthesis of bicyclic aminocyclopropanes by lanthanum-catalyzed asymmetric carboamination/annulation of cyclopropenes with aminoalkenes. This protocol features 100% atom efficiency, good yield (up to 90%), and high chemo- (up to >20:1) and stereoselectivity (up to >20:1 dr and 99% ee), constituting a unique route for the efficient synthesis of two different diastereoisomers of a given chiral bicyclic aminocyclopropane compound.
中文翻译:

手性镧催化剂的非对映异构不对称碳氨化/环丙烯与氨基烯烃的环化
立体发散性不对称催化是一项重要技术,可允许从同一组起始原料有效地获得具有多个立体中心的给定产品的各种立体异构体,但迄今为止,其在合成高应力环丙烷化合物中的应用仍未探索。我们在这里报告了镧催化的不对称碳氨化/环丙烯与氨基烯烃的环合反应,首次合成了双环氨基环丙烷的非对映异构对映选择性。该方案具有100%的原子效率,良好的收率(高达90%),高化学能(高达> 20:1)和立体选择性(高达> 20:1 dr和99%ee),构成了一种独特的制备方法有效合成给定手性双环氨基环丙烷化合物的两种不同的非对映异构体。
更新日期:2017-11-16
中文翻译:

手性镧催化剂的非对映异构不对称碳氨化/环丙烯与氨基烯烃的环化
立体发散性不对称催化是一项重要技术,可允许从同一组起始原料有效地获得具有多个立体中心的给定产品的各种立体异构体,但迄今为止,其在合成高应力环丙烷化合物中的应用仍未探索。我们在这里报告了镧催化的不对称碳氨化/环丙烯与氨基烯烃的环合反应,首次合成了双环氨基环丙烷的非对映异构对映选择性。该方案具有100%的原子效率,良好的收率(高达90%),高化学能(高达> 20:1)和立体选择性(高达> 20:1 dr和99%ee),构成了一种独特的制备方法有效合成给定手性双环氨基环丙烷化合物的两种不同的非对映异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号