当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and antifungal activity of novel oxazolidin-2-one-linked 1,2,3-triazole derivatives
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1039/c7md00442g Alejandra Ramírez-Villalva 1, 2, 3, 4, 5 , Davir González-Calderón 1, 2, 3, 4, 5 , Roxana I. Rojas-García 1, 2, 3, 4, 5 , Carlos González-Romero 1, 2, 3, 4, 5 , Joaquín Tamaríz-Mascarúa 1, 5, 6, 7, 8 , Macario Morales-Rodríguez 2, 3, 4, 5, 9 , Nieves Zavala-Segovia 4, 5, 10 , Aydeé Fuentes-Benítes 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1039/c7md00442g Alejandra Ramírez-Villalva 1, 2, 3, 4, 5 , Davir González-Calderón 1, 2, 3, 4, 5 , Roxana I. Rojas-García 1, 2, 3, 4, 5 , Carlos González-Romero 1, 2, 3, 4, 5 , Joaquín Tamaríz-Mascarúa 1, 5, 6, 7, 8 , Macario Morales-Rodríguez 2, 3, 4, 5, 9 , Nieves Zavala-Segovia 4, 5, 10 , Aydeé Fuentes-Benítes 1, 2, 3, 4, 5
Affiliation
|
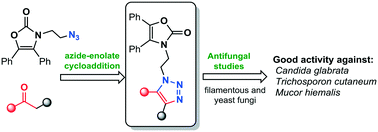
Novel oxazolidin-2-one-linked 1,2,3-triazole derivatives (4a–k) were synthesized by straightforward and versatile azide–enolate (3 + 2) cycloaddition. The series of compounds was screened for antifungal activity against four filamentous fungi as well as six yeast species of Candida spp. According to their efficiency and breadth of scope, they can be ordered as 4k > 4d > 4h > 4a, especially in relation to the activity displayed against Candida glabrata ATCC-34138, Trichosporon cutaneum ATCC-28592 and Mucor hiemalis ATCC-8690, i.e. compounds 4d, 4h and 4k showed excellent activity against C. glabrata (MIC 0.12, 0.25 and 0.12 μg mL−1, respectively), better than that of itraconazole (MIC 1 μg ml−1). The activity of compound 4d (MIC = 2 μg mL−1) was higher than that observed for the standard antifungal drug (MIC = 8 μg mL−1) against Trichosporon cutaneum, while compound 4k displayed an excellent antimycotic activity against Mucor hiemalis (MIC = 2 μg mL−1 vs. 4 μg mL−1 for itraconazole). In addition, we describe herein a novel mild and eco-friendly synthetic protocol for obtaining β-ketosulfones (adducts to afford compounds 4a–k) from α-brominated carbonyls in an aqueous nanomicellar medium at room temperature.
中文翻译:

恶唑烷-2-一键合1,2,3-三唑衍生物的合成及抗真菌活性
通过直接和通用的叠氮化物-烯酸酯(3 + 2)环加成反应合成了新型恶唑烷-2-酮连接的1,2,3-三唑衍生物(4a–k)。筛选了该系列化合物对四种丝状真菌和六种假丝酵母菌的抗真菌活性。根据它们的效率和范围广度,它们可以被排序为4K > 4D > 4H >图4A,特别是在相对于所述活动对显示光滑念珠菌ATCC-34138,皮状丝孢ATCC-28592和冻土毛霉ATCC-8690,即化合物4D,4h和4k显示出优异的抗光滑毛线虫的活性(分别为MIC 0.12、0.25和0.12μgmL -1),优于伊曲康唑(MIC 1μgml -1)。化合物4d(MIC = 2μgmL -1)的活性高于标准抗真菌药物(MIC = 8μgmL -1)对角皮癣菌的活性,而化合物4k表现出优异的抗毛孔霉菌(MIC)的抗霉菌活性。= 2μgmL -1 和4μgmL -1对于伊曲康唑)。另外,我们在本文中描述了一种新颖的温和且环保的合成方案,用于在室温下在水性纳米胶束介质中从α-溴代羰基获得β-酮砜(加成物,得到化合物4a-k)。
更新日期:2017-11-16
中文翻译:

恶唑烷-2-一键合1,2,3-三唑衍生物的合成及抗真菌活性
通过直接和通用的叠氮化物-烯酸酯(3 + 2)环加成反应合成了新型恶唑烷-2-酮连接的1,2,3-三唑衍生物(4a–k)。筛选了该系列化合物对四种丝状真菌和六种假丝酵母菌的抗真菌活性。根据它们的效率和范围广度,它们可以被排序为4K > 4D > 4H >图4A,特别是在相对于所述活动对显示光滑念珠菌ATCC-34138,皮状丝孢ATCC-28592和冻土毛霉ATCC-8690,即化合物4D,4h和4k显示出优异的抗光滑毛线虫的活性(分别为MIC 0.12、0.25和0.12μgmL -1),优于伊曲康唑(MIC 1μgml -1)。化合物4d(MIC = 2μgmL -1)的活性高于标准抗真菌药物(MIC = 8μgmL -1)对角皮癣菌的活性,而化合物4k表现出优异的抗毛孔霉菌(MIC)的抗霉菌活性。= 2μgmL -1 和4μgmL -1对于伊曲康唑)。另外,我们在本文中描述了一种新颖的温和且环保的合成方案,用于在室温下在水性纳米胶束介质中从α-溴代羰基获得β-酮砜(加成物,得到化合物4a-k)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号