当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanism of copper-catalyzed oxytrifluoromethylation of allylamines with CO2: a computational study
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7qo00838d Lei Zhu 1, 2, 3, 4 , Jian-Heng Ye 4, 5, 6, 7, 8 , Meng Duan 1, 2, 3, 4 , Xiaotian Qi 1, 2, 3, 4 , Da-Gang Yu 4, 5, 6, 7, 8 , Ruopeng Bai 1, 2, 3, 4 , Yu Lan 1, 2, 3, 4, 9
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7qo00838d Lei Zhu 1, 2, 3, 4 , Jian-Heng Ye 4, 5, 6, 7, 8 , Meng Duan 1, 2, 3, 4 , Xiaotian Qi 1, 2, 3, 4 , Da-Gang Yu 4, 5, 6, 7, 8 , Ruopeng Bai 1, 2, 3, 4 , Yu Lan 1, 2, 3, 4, 9
Affiliation
|
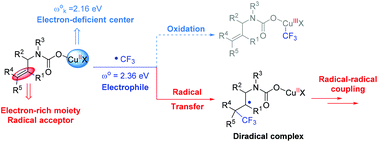
The trifluoromethyl group (CF3) is a very important functional group. The mechanism of transition metal catalyzed trifluoromethylation has received considerable attention in recent years. In this work, the detailed mechanism of the copper-catalyzed oxytrifluoromethylation of allylamines with CO2 was investigated by density functional theory (DFT) calculations. Differing from the previous Cu(I)–Cu(III) catalytic cycle, the results show that the reaction proceeds through a Cu(I)–Cu(II) catalytic cycle. Deprotonation of allylamines initially occurs with the assistance of a copper catalyst followed by CO2 insertion. In the presence of Togni reagent II, the copper(I) carboxylate species can then be oxidized to the copper(II) dicarboxylate intermediate along with the formation of a free trifluoromethyl radical, which then attacks the alkene moiety to generate the electrophilic addition diradical adduct. The spiro ring is constructed by a carboxylate-delivered radical–radical cross-coupling procedure. In addition, the calculated global electrophilicity shows that the copper(III) intermediate cannot be generated from the combination of the electron deficient copper center and the electrophilic trifluoromethyl radical. Frontier molecular orbital analysis indicates that the Togni reagent II is activated by neutral Cu(I) rather than the cationic Cu(I) species. The origin of the diastereoselectivity can be mainly attributed to the repulsion between the trifluoromethyl group and the carbonyl moiety.
中文翻译:

铜催化烯丙胺与CO 2的氧化三氟甲基化反应机理的计算研究
三氟甲基(CF 3)是非常重要的官能团。近年来,过渡金属催化三氟甲基化的机理受到了相当大的关注。在这项工作中,通过密度泛函理论(DFT)计算研究了铜胺与CO 2催化烯丙基胺的氧三氟甲基化的详细机理。与先前的Cu(I)–Cu(III)催化循环不同,结果表明反应通过Cu(I)–Cu(II)催化循环进行。烯丙基胺的去质子反应首先是在铜催化剂的辅助下进行,然后是CO 2插入。在存在Togni试剂II的情况下,然后可以将羧酸铜(I)物种氧化为二羧酸铜(II)中间体,同时形成游离的三氟甲基自由基,然后该自由基攻击烯烃部分以生成亲电子加成双自由基加合物。螺环是由羧酸盐传递的自由基与自由基的交叉偶联过程构成的。另外,计算的整体亲电性表明,不能从缺电子的铜中心和亲电三氟甲基自由基的组合中生成铜(III)中间体。前沿分子轨道分析表明,Togni试剂II被中性Cu(I)而不是阳离子Cu(I)物种。非对映选择性的起源可主要归因于三氟甲基和羰基部分之间的排斥。
更新日期:2017-11-16
中文翻译:

铜催化烯丙胺与CO 2的氧化三氟甲基化反应机理的计算研究
三氟甲基(CF 3)是非常重要的官能团。近年来,过渡金属催化三氟甲基化的机理受到了相当大的关注。在这项工作中,通过密度泛函理论(DFT)计算研究了铜胺与CO 2催化烯丙基胺的氧三氟甲基化的详细机理。与先前的Cu(I)–Cu(III)催化循环不同,结果表明反应通过Cu(I)–Cu(II)催化循环进行。烯丙基胺的去质子反应首先是在铜催化剂的辅助下进行,然后是CO 2插入。在存在Togni试剂II的情况下,然后可以将羧酸铜(I)物种氧化为二羧酸铜(II)中间体,同时形成游离的三氟甲基自由基,然后该自由基攻击烯烃部分以生成亲电子加成双自由基加合物。螺环是由羧酸盐传递的自由基与自由基的交叉偶联过程构成的。另外,计算的整体亲电性表明,不能从缺电子的铜中心和亲电三氟甲基自由基的组合中生成铜(III)中间体。前沿分子轨道分析表明,Togni试剂II被中性Cu(I)而不是阳离子Cu(I)物种。非对映选择性的起源可主要归因于三氟甲基和羰基部分之间的排斥。











































 京公网安备 11010802027423号
京公网安备 11010802027423号