当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
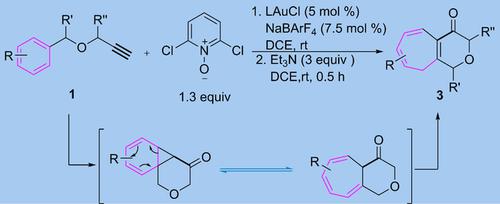
Cyclopropanation of Benzene Rings by Oxidatively Generated α-Oxo Gold Carbene: One-Pot Access to Tetrahydropyranone-Fused Cycloheptatrienes from Propargyl Benzyl Ethers.
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-14 , DOI: 10.1002/adsc.201701322 Kegong Ji 1, 2 , Liming Zhang 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-14 , DOI: 10.1002/adsc.201701322 Kegong Ji 1, 2 , Liming Zhang 2
Affiliation
|
Cyclopropanations of benzene rings by oxidatively generated α-oxo gold carbenes are for the first time demonstrated in a Buchner reaction, in which readily available propargyl benzyl ethers are converted in one-pot to tetrahydropyranone-fused cycloheptatrienes via sequential oxidative gold catalysis and base-promoted isomerization. Additional examples of arene cyclopropanations without fragmentation of the cyclopropane ring are also realized.
中文翻译:

氧化生成的α-氧代金碳烯对苯环的环丙烷化作用:一锅法从炔丙基苄基醚中获取四氢吡喃酮-熔合环庚烯。
在Buchner反应中首次证明了由氧化生成的α-氧代金卡宾对苯环的环丙烷化,其中易于获得的炔丙基苄基醚通过顺序氧化金催化作用,一锅转化为四氢吡喃酮稠合的环庚烯,并经碱促进异构化。还实现了芳烃环丙烷化而没有环丙烷环断裂的其他实例。
更新日期:2017-12-07
中文翻译:

氧化生成的α-氧代金碳烯对苯环的环丙烷化作用:一锅法从炔丙基苄基醚中获取四氢吡喃酮-熔合环庚烯。
在Buchner反应中首次证明了由氧化生成的α-氧代金卡宾对苯环的环丙烷化,其中易于获得的炔丙基苄基醚通过顺序氧化金催化作用,一锅转化为四氢吡喃酮稠合的环庚烯,并经碱促进异构化。还实现了芳烃环丙烷化而没有环丙烷环断裂的其他实例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号