当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Unexpected Domino Reaction of β-Keto Sulfones with Acetylene Ketones Promoted by Base: Facile Synthesis of 3(2H)-Furanones and Sulfonylbenzenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-15 12:31:14 , DOI: 10.1002/adsc.201700830 Wei Tong 1 , Qian-Yu Li 1 , Yan-Li Xu 2 , Heng-Shan Wang 1 , Yan-Yan Chen 2 , Ying-Ming Pan 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-15 12:31:14 , DOI: 10.1002/adsc.201700830 Wei Tong 1 , Qian-Yu Li 1 , Yan-Li Xu 2 , Heng-Shan Wang 1 , Yan-Yan Chen 2 , Ying-Ming Pan 1
Affiliation
|
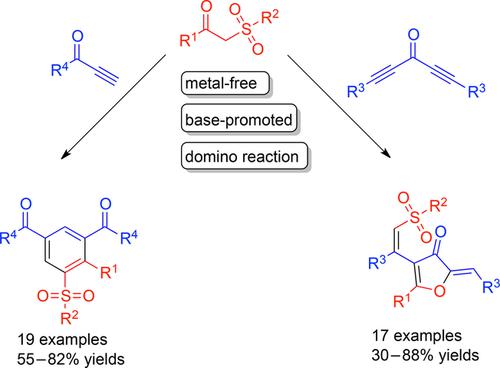
An unexpected domino reaction of β-keto sulfones with acetylene ketones has been developed. The domino reaction of β-keto sulfones with diynones proceeded smoothly in the presence of 30 mol% K2CO3 without other additives, and afforded the novel 3(2H)-furanone derivatives. On replacing the diynones with terminal alkyne ketones, the reaction regioselectivity was changed and sulfonylbenzenes were obtained via benzannulation in good yields.
中文翻译:

β-酮砜与碱促进的乙炔酮发生意外的多米诺反应:轻松合成3(2H)-呋喃酮和磺酰基苯
已经开发出β-酮砜与乙炔酮的出乎意料的多米诺骨牌反应。在没有其他添加剂的情况下,在30 mol%K 2 CO 3的存在下,β-酮砜与二炔酮的多米诺反应顺利进行,得到了新颖的3(2 H)-呋喃酮衍生物。用末端炔烃酮取代二炔酮后,反应区域选择性改变,并通过苯环化获得磺酰苯,收率很高。
更新日期:2017-11-16
中文翻译:

β-酮砜与碱促进的乙炔酮发生意外的多米诺反应:轻松合成3(2H)-呋喃酮和磺酰基苯
已经开发出β-酮砜与乙炔酮的出乎意料的多米诺骨牌反应。在没有其他添加剂的情况下,在30 mol%K 2 CO 3的存在下,β-酮砜与二炔酮的多米诺反应顺利进行,得到了新颖的3(2 H)-呋喃酮衍生物。用末端炔烃酮取代二炔酮后,反应区域选择性改变,并通过苯环化获得磺酰苯,收率很高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号