当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric 1,6-Conjugate Addition of para-Quinone Methides for the Synthesis of Chiral β,β-Diaryl-α-Hydroxy Ketones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-15 05:30:58 , DOI: 10.1002/adsc.201700947 Yuan-Yuan Gao 1 , Yuan-Zhao Hua 1 , Min-Can Wang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-15 05:30:58 , DOI: 10.1002/adsc.201700947 Yuan-Yuan Gao 1 , Yuan-Zhao Hua 1 , Min-Can Wang 1
Affiliation
|
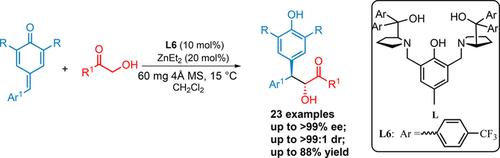
A direct asymmetric 1,6-conjugated addition of α-hydroxy ketone to para-quinone methides has been developed. It is an efficient approach to the synthesis of chiral β,β-diaryl-α-hydroxy ketones. The reaction runs smoothly in the presence of ZnEt2 and nonracemic bis(diarylhydroxymethylpyrrolidinylmethyl)phenols (ProPhenols), and the desired products are obtained in good yields (up to 88%) and with high stereoselectivity (up to >99% ee and up to >99:1 dr). This reaction also can be run on a gram scale without impacting its enantioselectivity.
中文翻译:

对苯二甲腈的不对称1,6-共轭加成反应合成手性β,β-二芳基-α-羟基酮
已经开发了α-羟基酮直接不对称的1,6-共轭加成到对醌甲基化物中。这是合成手性β,β-二芳基-α-羟基酮的有效方法。该反应在ZnEt 2和非外消旋双(二芳基羟甲基吡咯烷二甲基甲基)苯酚(ProPhenols)的存在下顺利进行,并以良好的收率(最高88%)和高的立体选择性(最高ee> 99%和最高ee)获得了所需的产物。 > 99:1博士)。该反应也可以以克规模进行,而不会影响其对映选择性。
更新日期:2017-11-16
中文翻译:

对苯二甲腈的不对称1,6-共轭加成反应合成手性β,β-二芳基-α-羟基酮
已经开发了α-羟基酮直接不对称的1,6-共轭加成到对醌甲基化物中。这是合成手性β,β-二芳基-α-羟基酮的有效方法。该反应在ZnEt 2和非外消旋双(二芳基羟甲基吡咯烷二甲基甲基)苯酚(ProPhenols)的存在下顺利进行,并以良好的收率(最高88%)和高的立体选择性(最高ee> 99%和最高ee)获得了所需的产物。 > 99:1博士)。该反应也可以以克规模进行,而不会影响其对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号