当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Shift as an Entropy-Driven Effect
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.biochem.7b00860 Martin Redhead 1 , Rupert Satchell 1 , Ciara McCarthy 1 , Scott Pollack 1 , John Unitt 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.biochem.7b00860 Martin Redhead 1 , Rupert Satchell 1 , Ciara McCarthy 1 , Scott Pollack 1 , John Unitt 1
Affiliation
|
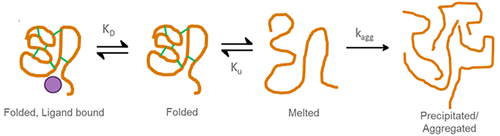
Thermal shift assays (TSAs) are among the most commonly used biophysical approaches in drug discovery in both academic and industrial settings. However, the most common interpretation of the data generated by a TSA is purely qualitative, using only the change in melting temperature (ΔTm) as a metric. This has left many questions surrounding the interpretation of the data as well as whether the TSA truly correlates with other assays. TSAs also lack theoretical descriptions of the melt behavior of proteins in the presence of multiple ligands. Here we describe a novel simplified analytical framework based on “pseudoisothermal” models as well as exact thermodynamic descriptions of protein–ligand melt behavior rooted in changes in the entropy of melting. We show how the models are broad and independently applicable, in that they can describe the behavior of any macromolecule such as a protein or DNA and demonstrate good correlations with other techniques. These models are shown to give good descriptions of assay systems containing single or multiple ligands and can determine the mechanism of interaction. The models are derived from first principles, and the theoretical justification is discussed.
中文翻译:

作为熵驱动效应的热位移
在学术和工业环境中,热位移分析(TSA)是药物发现中最常用的生物物理方法之一。但是,TSA生成的数据最常见的解释是纯定性的,仅使用熔化温度的变化(ΔT m)作为指标。这留下了许多围绕数据解释以及TSA是否与其他测定真正相关的问题。TSA还缺乏在多个配体存在下蛋白质解链行为的理论描述。在这里,我们描述了一种基于“伪等温”模型的新颖简化分析框架,以及基于融化熵变化的蛋白质-配体融化行为的精确热力学描述。我们展示了模型如何广泛和独立适用,因为它们可以描述任何大分子(例如蛋白质或DNA)的行为,并证明与其他技术的良好相关性。这些模型显示出对包含单个或多个配体的测定系统的良好描述,并且可以确定相互作用的机制。
更新日期:2017-11-16
中文翻译:

作为熵驱动效应的热位移
在学术和工业环境中,热位移分析(TSA)是药物发现中最常用的生物物理方法之一。但是,TSA生成的数据最常见的解释是纯定性的,仅使用熔化温度的变化(ΔT m)作为指标。这留下了许多围绕数据解释以及TSA是否与其他测定真正相关的问题。TSA还缺乏在多个配体存在下蛋白质解链行为的理论描述。在这里,我们描述了一种基于“伪等温”模型的新颖简化分析框架,以及基于融化熵变化的蛋白质-配体融化行为的精确热力学描述。我们展示了模型如何广泛和独立适用,因为它们可以描述任何大分子(例如蛋白质或DNA)的行为,并证明与其他技术的良好相关性。这些模型显示出对包含单个或多个配体的测定系统的良好描述,并且可以确定相互作用的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号