当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Conformational Dynamics in the Evolution of Retro-Aldolase Activity
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acscatal.7b02954 Adrian Romero-Rivera 1 , Marc Garcia-Borràs 1, 2 , Sílvia Osuna 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acscatal.7b02954 Adrian Romero-Rivera 1 , Marc Garcia-Borràs 1, 2 , Sílvia Osuna 1
Affiliation
|
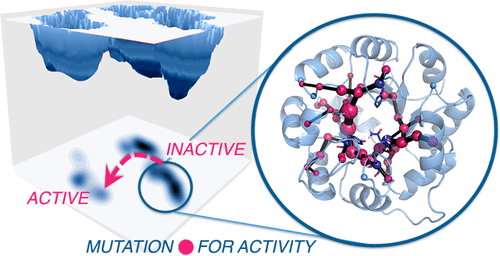
Enzymes exist as ensembles of conformations that are important for function. Tuning these populations of conformational states through mutation enables evolution toward additional activities. Here we computationally evaluate the population shifts induced by distal and active site mutations in a family of computationally designed and experimentally optimized retro-aldolases. The conformational landscape of these enzymes was significantly altered during evolution, as pre-existing catalytically active conformational substates became major states in the most evolved variants. We further demonstrate that key residues responsible for these substate conversions can be predicted computationally. Significantly, the identified residues coincide with those positions mutated in the laboratory evolution experiments. This study establishes that distal mutations that affect enzyme catalytic activity can be predicted computationally and thus provides the enzyme (re)design field with a rational strategy to determine promising sites for enhancing activity through mutation.
中文翻译:

构象动力学在逆醛缩酶活性演变中的作用
酶以对功能重要的构象形式存在。通过突变调整这些构象状态的种群,可以朝着其他活动的方向发展。在这里,我们在计算设计和实验优化的逆醛糖酶家族中,通过计算来评估由远端和活动位点突变引起的种群转移。这些酶的构象态势在进化过程中发生了显着变化,因为预先存在的催化活性构象亚状态成为大多数进化变体中的主要状态。我们进一步证明负责这些子状态转换的关键残基可以通过计算来预测。重要的是,鉴定出的残基与实验室进化实验中突变的那些位置一致。
更新日期:2017-11-16
中文翻译:

构象动力学在逆醛缩酶活性演变中的作用
酶以对功能重要的构象形式存在。通过突变调整这些构象状态的种群,可以朝着其他活动的方向发展。在这里,我们在计算设计和实验优化的逆醛糖酶家族中,通过计算来评估由远端和活动位点突变引起的种群转移。这些酶的构象态势在进化过程中发生了显着变化,因为预先存在的催化活性构象亚状态成为大多数进化变体中的主要状态。我们进一步证明负责这些子状态转换的关键残基可以通过计算来预测。重要的是,鉴定出的残基与实验室进化实验中突变的那些位置一致。







































 京公网安备 11010802027423号
京公网安备 11010802027423号