当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Defining the domains of Cia2 required for its essential function in vivo and in vitro
Metallomics ( IF 2.9 ) Pub Date : 2017-08-23 00:00:00 , DOI: 10.1039/c7mt00181a Amanda T. Vo 1, 2, 3, 4 , Nicholas M. Fleischman 1, 2, 3, 4 , Melissa D. Marquez 1, 2, 3, 4 , Eric J. Camire 1, 2, 3, 4 , Stephanie U. Esonwune 1, 2, 3, 4 , John D. Grossman 1, 2, 3, 4 , Kelly A. Gay 1, 2, 3, 4 , Jessica A. Cosman 1, 2, 3, 4 , Deborah L. Perlstein 1, 2, 3, 4
Metallomics ( IF 2.9 ) Pub Date : 2017-08-23 00:00:00 , DOI: 10.1039/c7mt00181a Amanda T. Vo 1, 2, 3, 4 , Nicholas M. Fleischman 1, 2, 3, 4 , Melissa D. Marquez 1, 2, 3, 4 , Eric J. Camire 1, 2, 3, 4 , Stephanie U. Esonwune 1, 2, 3, 4 , John D. Grossman 1, 2, 3, 4 , Kelly A. Gay 1, 2, 3, 4 , Jessica A. Cosman 1, 2, 3, 4 , Deborah L. Perlstein 1, 2, 3, 4
Affiliation
|
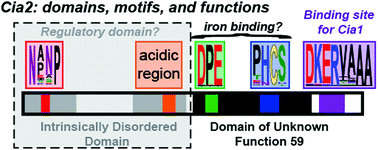
The cytosolic iron–sulfur cluster assembly (CIA) system biosynthesizes iron–sulfur (FeS) cluster cofactors for cytosolic and nuclear proteins. The yeast Cia2 protein is the central component of the targeting complex which identifies apo-protein targets in the final step of the pathway. Herein, we determine that Cia2 contains five conserved motifs distributed between an intrinsically disordered N-terminal domain and a C-terminal domain of unknown function 59 (DUF59). The disordered domain is dispensible for binding the other subunits of the targeting complex, Met18 and Cia1, and the apo-target Rad3 in vitro. While in vivo assays reveal that the C-terminal domain is sufficient to support viability, several phenotypic assays indicate that deletion of the N-terminal domain negatively impacts CIA function. We additionally establish that Glu208, located within a conserved motif found only in eukaryotic DUF59 proteins, is important for the Cia1–Cia2 interaction in vitro. In vivo, E208A–Cia2 results in a diminished activity of the cytosolic iron sulfur cluster protein, Leu1 but only modest effects on hydroxyurea or methylmethane sulfonate sensitivity. Finally, we demonstrate that neither of the two highly conserved motifs of the DUF59 domain are vital for any of Cia2's interactions in vitro yet mutation of the DPE motif in the DUF59 domain results in a nonfunctional allele in vivo. Our observation that four of the five highly conserved motifs of Cia2 are dispensable for targeting complex formation and apo-target binding suggests that Cia2 is not simply a protein–protein interaction mediator but it likely possesses an additional, currently cryptic, function during the final cluster insertion step of CIA.
中文翻译:

定义Cia2在体内和体外的基本功能所需的域
胞质铁硫簇装配(CIA)系统可生物合成胞质和核蛋白的铁硫(FeS)簇辅助因子。酵母Cia2蛋白是靶向复合物的主要成分,该复合物可在途径的最后一步识别载脂蛋白靶标。在本文中,我们确定Cia2包含五个保守的基序,分布在本质上无序的N末端结构域和未知功能59(DUF59)的C末端结构域之间。无序域对于在体外结合靶向复合物的其他亚基Met18和Cia1以及脱辅基靶Rad3而言是必不可少的。虽然在体内分析表明C端结构域足以支持生存力,几种表型分析表明N端结构域的缺失对CIA功能产生负面影响。我们还确定,位于仅在真核DUF59蛋白中发现的保守基序内的Glu208对于Cia1–Cia2体外相互作用很重要。在体内,E208A–Cia2导致胞质铁硫簇蛋白Leu1的活性降低,但对羟基脲或甲基甲烷磺酸盐敏感性的影响很小。最后,我们证明了DUF59域的两个高度保守的基序都不对任何Cia2的体外相互作用都至关重要,但DUF59域中DPE基序的突变会导致无功能的等位基因体内。我们的观察发现,Cia2的五个高度保守的基序中的四个对于靶向复合物的形成和载脂蛋白-靶标结合而言是可有可无的,这表明Cia2不仅是蛋白质-蛋白质相互作用的介体,而且在最终簇中可能还具有额外的(目前是隐秘的)功能CIA的插入步骤。
更新日期:2017-11-16
中文翻译:

定义Cia2在体内和体外的基本功能所需的域
胞质铁硫簇装配(CIA)系统可生物合成胞质和核蛋白的铁硫(FeS)簇辅助因子。酵母Cia2蛋白是靶向复合物的主要成分,该复合物可在途径的最后一步识别载脂蛋白靶标。在本文中,我们确定Cia2包含五个保守的基序,分布在本质上无序的N末端结构域和未知功能59(DUF59)的C末端结构域之间。无序域对于在体外结合靶向复合物的其他亚基Met18和Cia1以及脱辅基靶Rad3而言是必不可少的。虽然在体内分析表明C端结构域足以支持生存力,几种表型分析表明N端结构域的缺失对CIA功能产生负面影响。我们还确定,位于仅在真核DUF59蛋白中发现的保守基序内的Glu208对于Cia1–Cia2体外相互作用很重要。在体内,E208A–Cia2导致胞质铁硫簇蛋白Leu1的活性降低,但对羟基脲或甲基甲烷磺酸盐敏感性的影响很小。最后,我们证明了DUF59域的两个高度保守的基序都不对任何Cia2的体外相互作用都至关重要,但DUF59域中DPE基序的突变会导致无功能的等位基因体内。我们的观察发现,Cia2的五个高度保守的基序中的四个对于靶向复合物的形成和载脂蛋白-靶标结合而言是可有可无的,这表明Cia2不仅是蛋白质-蛋白质相互作用的介体,而且在最终簇中可能还具有额外的(目前是隐秘的)功能CIA的插入步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号