Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Varying Doses of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Exposure Among Patients With Peanut Sensitivity
JAMA ( IF 63.1 ) Pub Date : 2017-11-14 , DOI: 10.1001/jama.2017.16591 Hugh A. Sampson 1 , Wayne G. Shreffler 2 , William H. Yang 3 , Gordon L. Sussman 4 , Terri F. Brown-Whitehorn 5 , Kari C. Nadeau 6 , Amarjit S. Cheema 7 , Stephanie A. Leonard 8 , Jacqueline A. Pongracic 9 , Christine Sauvage-Delebarre 10 , Amal H. Assa’ad 11 , Frederic de Blay 12 , J. Andrew Bird 13 , Stephen A. Tilles 14 , Franck Boralevi 15 , Thierry Bourrier 16 , Jacques Hébert 17 , Todd D. Green 18 , Roy Gerth van Wijk 19 , André C. Knulst 20 , Gisèle Kanny 21 , Lynda C. Schneider 22 , Marek L. Kowalski 23 , Christophe Dupont 24
JAMA ( IF 63.1 ) Pub Date : 2017-11-14 , DOI: 10.1001/jama.2017.16591 Hugh A. Sampson 1 , Wayne G. Shreffler 2 , William H. Yang 3 , Gordon L. Sussman 4 , Terri F. Brown-Whitehorn 5 , Kari C. Nadeau 6 , Amarjit S. Cheema 7 , Stephanie A. Leonard 8 , Jacqueline A. Pongracic 9 , Christine Sauvage-Delebarre 10 , Amal H. Assa’ad 11 , Frederic de Blay 12 , J. Andrew Bird 13 , Stephen A. Tilles 14 , Franck Boralevi 15 , Thierry Bourrier 16 , Jacques Hébert 17 , Todd D. Green 18 , Roy Gerth van Wijk 19 , André C. Knulst 20 , Gisèle Kanny 21 , Lynda C. Schneider 22 , Marek L. Kowalski 23 , Christophe Dupont 24
Affiliation
|
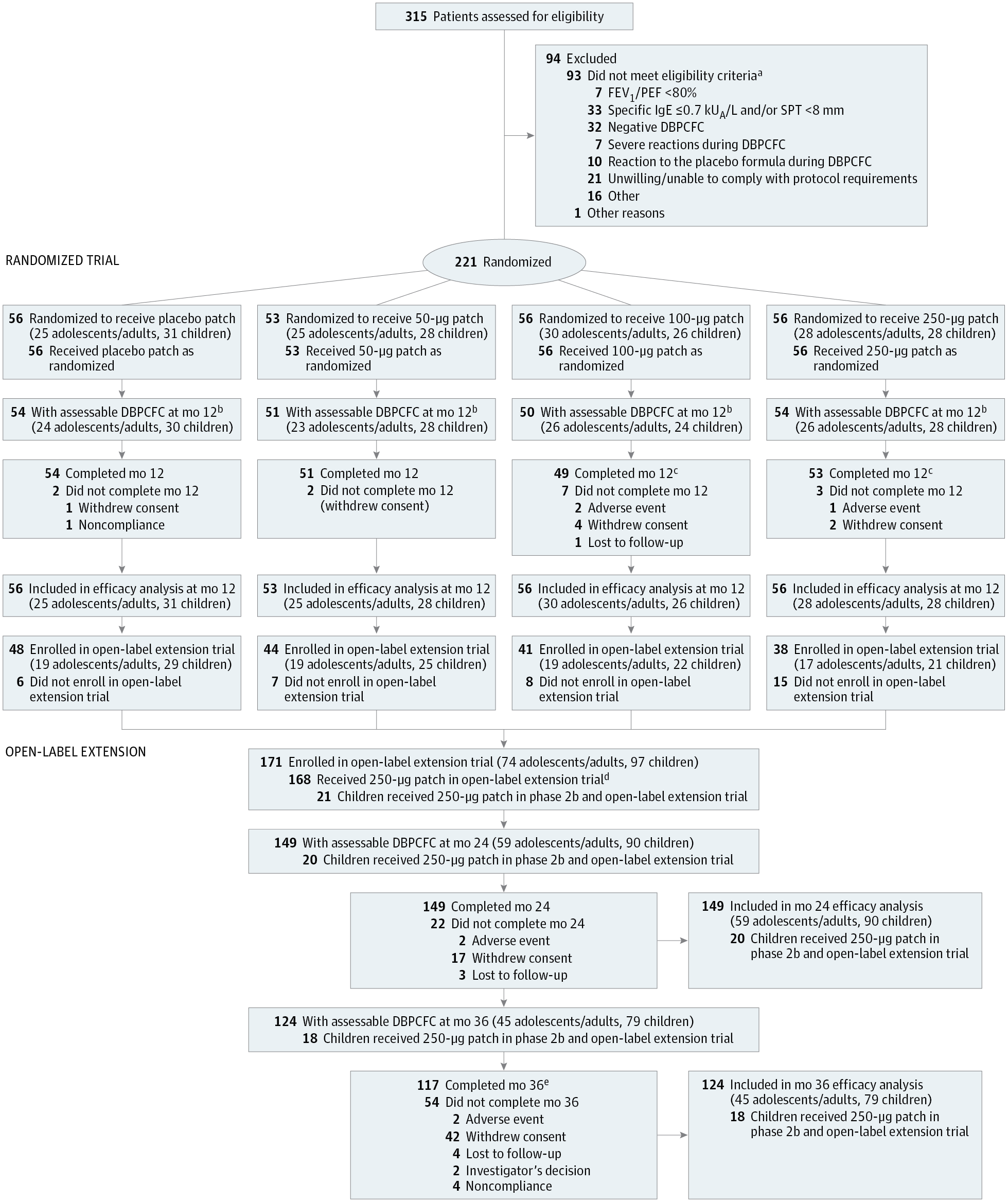
Importance Epicutaneous immunotherapy may have potential for treating peanut allergy but has been assessed only in preclinical and early human trials. Objective To determine the optimal dose, adverse events (AEs), and efficacy of a peanut patch for peanut allergy treatment. Design, Setting, and Participants Phase 2b double-blind, placebo-controlled, dose-ranging trial of a peanut patch in peanut-allergic patients (6-55 years) from 22 centers, with a 2-year, open-label extension (July 31, 2012-July 31, 2014; extension completed September 29, 2016). Patients (n = 221) had peanut sensitivity and positive double-blind, placebo-controlled food challenges to an eliciting dose of 300 mg or less of peanut protein. Interventions Randomly assigned patients (1:1:1:1) received an epicutaneous peanut patch containing 50 &mgr;g (n = 53), 100 &mgr;g (n = 56), or 250 &mgr;g (n = 56) of peanut protein or a placebo patch (n = 56). Following daily patch application for 12 months, patients underwent a double-blind, placebo-controlled food challenge to establish changes in eliciting dose. Main Outcomes and Measures The primary efficacy end point was percentage of treatment responders (eliciting dose: ≥10-times increase and/or reaching ≥1000 mg of peanut protein) in each group vs placebo patch after 12 months. Secondary end points included percentage of responders by age strata and treatment-emergent adverse events (TEAEs). Results Of 221 patients randomized (median age, 11 years [quartile 1, quartile 3: 8, 16]; 37.6% female), 93.7% completed the trial. A significant absolute difference in response rates was observed at month 12 between the 250-&mgr;g (n = 28; 50.0%) and placebo (n = 14; 25.0%) patches (difference, 25.0%; 95% CI, 7.7%-42.3%; P = .01). No significant difference was seen between the placebo patch vs the 100-&mgr;g patch. Because of statistical testing hierarchical rules, the 50-&mgr;g patch was not compared with placebo. Interaction by age group was only significant for the 250-&mgr;g patch (P = .04). In the 6- to 11-year stratum, the response rate difference between the 250-&mgr;g (n = 15; 53.6%) and placebo (n = 6; 19.4%) patches was 34.2% (95% CI, 11.1%-57.3%; P = .008); adolescents/adults showed no difference between the 250-&mgr;g (n = 13; 46.4%) and placebo (n = 8; 32.0%) patches: 14.4% (95% CI, −11.6% to 40.4%; P = .40). No dose-related serious AEs were observed. The percentage of patients with 1 or more TEAEs (largely local skin reactions) was similar across all groups in year 1: 50-&mgr;g patch = 100%, 100-&mgr;g patch = 98.2%, 250-&mgr;g patch = 100%, and placebo patch = 92.9%. The overall median adherence was 97.6% after 1 year; the dropout rate for treatment-related AEs was 0.9%. Conclusions and Relevance In this dose-ranging trial of peanut-allergic patients, the 250-&mgr;g peanut patch resulted in significant treatment response vs placebo patch following 12 months of therapy. These findings warrant a phase 3 trial. Trial Registration clinicaltrials.gov Identifier: NCT01675882
中文翻译:

不同剂量的表皮免疫疗法与安慰剂对花生过敏患者对花生蛋白暴露反应的影响
重要性 表皮免疫疗法可能具有治疗花生过敏的潜力,但仅在临床前和早期人体试验中进行了评估。目的 确定花生贴剂治疗花生过敏的最佳剂量、不良事件 (AE) 和疗效。设计、设置和参与者 2b 期双盲、安慰剂对照、剂量范围试验,对来自 22 个中心的花生过敏患者(6-55 岁)进行了花生贴片,为期 2 年,开放标签扩展( 2012年7月31日-2014年7月31日;延期于2016年9月29日完成)。患者 (n = 221) 对花生蛋白敏感,并且对 300 毫克或更少花生蛋白的诱导剂量进行了双盲、安慰剂对照的阳性食物激发试验。干预 随机分配的患者 (1:1:1:1) 接受含有 50 &mgr;g (n = 53)、100 &mgr; g (n = 56) 或 250 μg (n = 56) 的花生蛋白或安慰剂贴剂 (n = 56)。在每日贴剂应用 12 个月后,患者接受了双盲、安慰剂对照的食物激发试验,以确定诱发剂量的变化。主要结果和测量 主要疗效终点是 12 个月后每组与安慰剂贴剂的治疗反应者百分比(引发剂量:增加≥10 倍和/或达到≥1000 mg 花生蛋白)。次要终点包括按年龄层和治疗出现的不良事件 (TEAE) 划分的应答者百分比。结果 在随机分配的 221 名患者中(中位年龄,11 岁 [四分位数 1,四分位数 3:8、16];37.6% 为女性),93.7% 的患者完成了试验。在第 12 个月观察到 250-&mgr;g(n = 28;50.0%)和安慰剂(n = 14;25.0%) 补丁(差异,25.0%;95% CI,7.7%-42.3%;P = .01)。安慰剂贴剂与 100-&mgr;g 贴剂之间没有发现显着差异。由于统计检验分级规则,50-&mgr;g 贴剂未与安慰剂进行比较。年龄组的相互作用仅对 250-&mgr;g 贴剂显着(P = .04)。在 6 到 11 年的层次中,250-&mgr;g(n = 15;53.6%)和安慰剂(n = 6;19.4%)贴剂之间的反应率差异为 34.2%(95% CI,11.1%) -57.3%;P = .008);青少年/成人在 250-&mgr;g(n = 13;46.4%)和安慰剂(n = 8;32.0%)贴片之间没有差异:14.4%(95% CI,-11.6% 至 40.4%;P = . 40)。没有观察到剂量相关的严重 AE。第 1 年所有组中出现 1 个或多个 TEAE(主要是局部皮肤反应)的患者百分比相似:50-& mgr;g 贴片 = 100%,100-&mgr;g 贴片 = 98.2%,250-&mgr;g 贴片 = 100%,安慰剂贴片 = 92.9%。1 年后的总体中位依从率为 97.6%;治疗相关 AE 的退出率为 0.9%。结论和相关性 在这项针对花生过敏患者的剂量范围试验中,在 12 个月的治疗后,250-g 花生贴剂与安慰剂贴剂相比产生了显着的治疗反应。这些发现值得进行 3 期试验。试验注册clinicaltrials.gov 标识符:NCT01675882 在 12 个月的治疗后,g 花生贴片与安慰剂贴片相比产生了显着的治疗反应。这些发现值得进行 3 期试验。试验注册clinicaltrials.gov 标识符:NCT01675882 在 12 个月的治疗后,g 花生贴片与安慰剂贴片相比产生了显着的治疗反应。这些发现值得进行 3 期试验。试验注册clinicaltrials.gov 标识符:NCT01675882
更新日期:2017-11-14
中文翻译:

不同剂量的表皮免疫疗法与安慰剂对花生过敏患者对花生蛋白暴露反应的影响
重要性 表皮免疫疗法可能具有治疗花生过敏的潜力,但仅在临床前和早期人体试验中进行了评估。目的 确定花生贴剂治疗花生过敏的最佳剂量、不良事件 (AE) 和疗效。设计、设置和参与者 2b 期双盲、安慰剂对照、剂量范围试验,对来自 22 个中心的花生过敏患者(6-55 岁)进行了花生贴片,为期 2 年,开放标签扩展( 2012年7月31日-2014年7月31日;延期于2016年9月29日完成)。患者 (n = 221) 对花生蛋白敏感,并且对 300 毫克或更少花生蛋白的诱导剂量进行了双盲、安慰剂对照的阳性食物激发试验。干预 随机分配的患者 (1:1:1:1) 接受含有 50 &mgr;g (n = 53)、100 &mgr; g (n = 56) 或 250 μg (n = 56) 的花生蛋白或安慰剂贴剂 (n = 56)。在每日贴剂应用 12 个月后,患者接受了双盲、安慰剂对照的食物激发试验,以确定诱发剂量的变化。主要结果和测量 主要疗效终点是 12 个月后每组与安慰剂贴剂的治疗反应者百分比(引发剂量:增加≥10 倍和/或达到≥1000 mg 花生蛋白)。次要终点包括按年龄层和治疗出现的不良事件 (TEAE) 划分的应答者百分比。结果 在随机分配的 221 名患者中(中位年龄,11 岁 [四分位数 1,四分位数 3:8、16];37.6% 为女性),93.7% 的患者完成了试验。在第 12 个月观察到 250-&mgr;g(n = 28;50.0%)和安慰剂(n = 14;25.0%) 补丁(差异,25.0%;95% CI,7.7%-42.3%;P = .01)。安慰剂贴剂与 100-&mgr;g 贴剂之间没有发现显着差异。由于统计检验分级规则,50-&mgr;g 贴剂未与安慰剂进行比较。年龄组的相互作用仅对 250-&mgr;g 贴剂显着(P = .04)。在 6 到 11 年的层次中,250-&mgr;g(n = 15;53.6%)和安慰剂(n = 6;19.4%)贴剂之间的反应率差异为 34.2%(95% CI,11.1%) -57.3%;P = .008);青少年/成人在 250-&mgr;g(n = 13;46.4%)和安慰剂(n = 8;32.0%)贴片之间没有差异:14.4%(95% CI,-11.6% 至 40.4%;P = . 40)。没有观察到剂量相关的严重 AE。第 1 年所有组中出现 1 个或多个 TEAE(主要是局部皮肤反应)的患者百分比相似:50-& mgr;g 贴片 = 100%,100-&mgr;g 贴片 = 98.2%,250-&mgr;g 贴片 = 100%,安慰剂贴片 = 92.9%。1 年后的总体中位依从率为 97.6%;治疗相关 AE 的退出率为 0.9%。结论和相关性 在这项针对花生过敏患者的剂量范围试验中,在 12 个月的治疗后,250-g 花生贴剂与安慰剂贴剂相比产生了显着的治疗反应。这些发现值得进行 3 期试验。试验注册clinicaltrials.gov 标识符:NCT01675882 在 12 个月的治疗后,g 花生贴片与安慰剂贴片相比产生了显着的治疗反应。这些发现值得进行 3 期试验。试验注册clinicaltrials.gov 标识符:NCT01675882 在 12 个月的治疗后,g 花生贴片与安慰剂贴片相比产生了显着的治疗反应。这些发现值得进行 3 期试验。试验注册clinicaltrials.gov 标识符:NCT01675882











































 京公网安备 11010802027423号
京公网安备 11010802027423号