当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of point mutations on the ultrafast photo-isomerization of Anabaena sensory rhodopsin
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-11-15 , DOI: 10.1039/c7fd00200a D. Agathangelou 1, 2, 3, 4, 5 , Y. Orozco-Gonzalez 1, 2, 3, 4, 5 , M. del Carmen Marín 6, 7, 8, 9 , P. P. Roy 10, 11, 12, 13 , J. Brazard 1, 2, 3, 4, 5 , H. Kandori 14, 15, 16, 17 , K.-H. Jung 18, 19, 20, 21, 22 , J. Léonard 1, 2, 3, 4, 5 , T. Buckup 10, 11, 12, 13 , N. Ferré 2, 5, 23, 24, 25 , M. Olivucci 6, 7, 8, 9, 26 , S. Haacke 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-11-15 , DOI: 10.1039/c7fd00200a D. Agathangelou 1, 2, 3, 4, 5 , Y. Orozco-Gonzalez 1, 2, 3, 4, 5 , M. del Carmen Marín 6, 7, 8, 9 , P. P. Roy 10, 11, 12, 13 , J. Brazard 1, 2, 3, 4, 5 , H. Kandori 14, 15, 16, 17 , K.-H. Jung 18, 19, 20, 21, 22 , J. Léonard 1, 2, 3, 4, 5 , T. Buckup 10, 11, 12, 13 , N. Ferré 2, 5, 23, 24, 25 , M. Olivucci 6, 7, 8, 9, 26 , S. Haacke 1, 2, 3, 4, 5
Affiliation
|
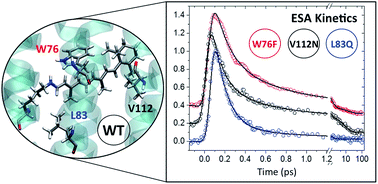
Anabaena sensory rhodopsin (ASR) is a particular microbial retinal protein for which light-adaptation leads to the ability to bind both the all-trans, 15-anti (AT) and the 13-cis, 15-syn (13C) isomers of the protonated Schiff base of retinal (PSBR). In the context of obtaining insight into the mechanisms by which retinal proteins catalyse the PSBR photo-isomerization reaction, ASR is a model system allowing to study, within the same protein, the protein–PSBR interactions for two different PSBR conformers at the same time. A detailed analysis of the vibrational spectra of AT and 13C, and their photo-products in wild-type ASR obtained through femtosecond (pump-) four-wave-mixing is reported for the first time, and compared to bacterio- and channelrhodopsin. As part of an extensive study of ASR mutants with blue-shifted absorption spectra, we present here a detailed computational analysis of the origin of the mutation-induced blue-shift of the absorption spectra, and identify electrostatic interactions as dominating steric effects that would entail a red-shift. The excited state lifetimes and isomerization reaction times (IRT) for the three mutants V112N, W76F, and L83Q are studied experimentally by femtosecond broadband transient absorption spectroscopy. Interestingly, in all three mutants, isomerization is accelerated for AT with respect to wild-type ASR, and this the more, the shorter the wavelength of maximum absorption. On the contrary, the 13C photo-reaction is slightly slowed down, leading to an inversion of the ESLs of AT and 13C, with respect to wt-ASR, in the blue-most absorbing mutant L83Q. Possible mechanisms for these mutation effects, and their steric and electrostatic origins are discussed.
中文翻译:

点突变对鱼腥藻视紫红质超快光异构化的影响
鱼腥藻视紫红质(ASR)是一种特殊的微生物视网膜蛋白,其光适应性使其能够结合全反式15抗(AT)和13-顺式15- syn(13C)视网膜质子化席夫碱(PSBR)的异构体。在深入了解视网膜蛋白催化PSBR光异构化反应的机理的背景下,ASR是一个模型系统,可以在同一蛋白内同时研究两个不同PSBR构象异构体的蛋白质-PSBR相互作用。首次详细报道了AT和13C的振动谱,以及通过飞秒(泵)四波混合获得的野生型ASR中的光产物,并将其与细菌视紫红质和通道视紫红质进行了比较。作为对具有蓝移吸收光谱的ASR突变体进行广泛研究的一部分,我们在此提供对由突变引起的吸收光谱蓝移的起源的详细计算分析,并确定静电相互作用是占主导地位的空间效应,这将引起红移。通过飞秒宽带瞬态吸收光谱实验研究了三个突变体V112N,W76F和L83Q的激发态寿命和异构化反应时间(IRT)。有趣的是,在所有三个突变体中,相对于野生型ASR,AT的异构化都得到了加速,而且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。通过飞秒宽带瞬态吸收光谱实验研究了三个突变体V112N,W76F和L83Q的激发态寿命和异构化反应时间(IRT)。有趣的是,在所有三个突变体中,相对于野生型ASR,AT的异构化都得到了加速,而且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。通过飞秒宽带瞬态吸收光谱实验研究了三个突变体V112N,W76F和L83Q的激发态寿命和异构化反应时间(IRT)。有趣的是,在所有三个突变体中,相对于野生型ASR,AT的异构化都得到了加速,而且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。相对于野生型ASR,AT的异构化得到了加速,并且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。相对于野生型ASR,AT的异构化得到了加速,并且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。
更新日期:2018-04-17
中文翻译:

点突变对鱼腥藻视紫红质超快光异构化的影响
鱼腥藻视紫红质(ASR)是一种特殊的微生物视网膜蛋白,其光适应性使其能够结合全反式15抗(AT)和13-顺式15- syn(13C)视网膜质子化席夫碱(PSBR)的异构体。在深入了解视网膜蛋白催化PSBR光异构化反应的机理的背景下,ASR是一个模型系统,可以在同一蛋白内同时研究两个不同PSBR构象异构体的蛋白质-PSBR相互作用。首次详细报道了AT和13C的振动谱,以及通过飞秒(泵)四波混合获得的野生型ASR中的光产物,并将其与细菌视紫红质和通道视紫红质进行了比较。作为对具有蓝移吸收光谱的ASR突变体进行广泛研究的一部分,我们在此提供对由突变引起的吸收光谱蓝移的起源的详细计算分析,并确定静电相互作用是占主导地位的空间效应,这将引起红移。通过飞秒宽带瞬态吸收光谱实验研究了三个突变体V112N,W76F和L83Q的激发态寿命和异构化反应时间(IRT)。有趣的是,在所有三个突变体中,相对于野生型ASR,AT的异构化都得到了加速,而且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。通过飞秒宽带瞬态吸收光谱实验研究了三个突变体V112N,W76F和L83Q的激发态寿命和异构化反应时间(IRT)。有趣的是,在所有三个突变体中,相对于野生型ASR,AT的异构化都得到了加速,而且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。通过飞秒宽带瞬态吸收光谱实验研究了三个突变体V112N,W76F和L83Q的激发态寿命和异构化反应时间(IRT)。有趣的是,在所有三个突变体中,相对于野生型ASR,AT的异构化都得到了加速,而且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。相对于野生型ASR,AT的异构化得到了加速,并且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。相对于野生型ASR,AT的异构化得到了加速,并且最大吸收的波长越短,异构化就越快。相反,在吸收最蓝的突变体L83Q中,相对于wt-ASR,13C的光反应稍微减慢,导致AT和13C的ESL反转。讨论了这些突变效应的可能机制,以及它们的空间和静电起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号