当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Deoxygenative [4+1] Annulation Involving N-Acyldiazenes for an Efficient Synthesis of 2,2,5-Trisubstituted 1,3,4-Oxadiazole Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-14 12:51:37 , DOI: 10.1002/adsc.201700935 Rong Zhou 1 , Ling Han 1 , Honghui Zhang 1 , Rongfang Liu 1 , Ruifeng Li 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-14 12:51:37 , DOI: 10.1002/adsc.201700935 Rong Zhou 1 , Ling Han 1 , Honghui Zhang 1 , Rongfang Liu 1 , Ruifeng Li 1
Affiliation
|
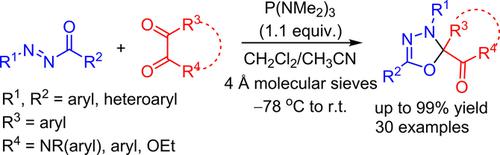
An unprecedented and highly efficient tris(dimethylamino)phosphine [P(NMe2)3]-mediated deoxygenative [4 + 1] annulation of N-acyldiazenes with α-dicarbonyl compounds such as isatins, α-keto esters, and α-diketones is reported. The annulation reactions proceed smoothly under mild conditions to deliver a broad range of 2,2,5-trisubstituted 1,3,4-oxadiazole derivatives in moderate to excellent yields from readily available starting materials. It represents the first realization of the [4+1] annulation mode involving N-acyldiazenes to construct five-membered heterocycles.
中文翻译:

涉及N-酰基二氮烯的高效合成2,2,5-三取代的1,3,4-Oxadiazole衍生物的脱氧[4 + 1]环。
前所未有的,高效的三(二甲基氨基)膦[P(NMe 2)3 ]介导的N-酰基二氮烯与α-二羰基化合物(如靛红,α-酮酸酯和α-二酮)的脱氧[4 +1]环化反应报告。在温和的条件下,环化反应可顺利进行,从容易获得的起始原料中以中等至极好的收率提供各种2,2,5-三取代的1,3,4-恶二唑衍生物。它代表了[4 + 1]环化模式的第一个实现,该模式涉及N-酰基二氮烯以构建五元杂环。
更新日期:2017-11-15
中文翻译:

涉及N-酰基二氮烯的高效合成2,2,5-三取代的1,3,4-Oxadiazole衍生物的脱氧[4 + 1]环。
前所未有的,高效的三(二甲基氨基)膦[P(NMe 2)3 ]介导的N-酰基二氮烯与α-二羰基化合物(如靛红,α-酮酸酯和α-二酮)的脱氧[4 +1]环化反应报告。在温和的条件下,环化反应可顺利进行,从容易获得的起始原料中以中等至极好的收率提供各种2,2,5-三取代的1,3,4-恶二唑衍生物。它代表了[4 + 1]环化模式的第一个实现,该模式涉及N-酰基二氮烯以构建五元杂环。









































 京公网安备 11010802027423号
京公网安备 11010802027423号