当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Synthesis of Selenide Ethers through a Decarboxylative Coupling Reaction
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-14 12:51:20 , DOI: 10.1002/adsc.201700676 Fei-Hu Cui 1 , Jing Chen 1 , Shi-Xia Su 1 , Yan-li Xu 2 , Heng-shan Wang 1 , Ying-ming Pan 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-14 12:51:20 , DOI: 10.1002/adsc.201700676 Fei-Hu Cui 1 , Jing Chen 1 , Shi-Xia Su 1 , Yan-li Xu 2 , Heng-shan Wang 1 , Ying-ming Pan 1
Affiliation
|
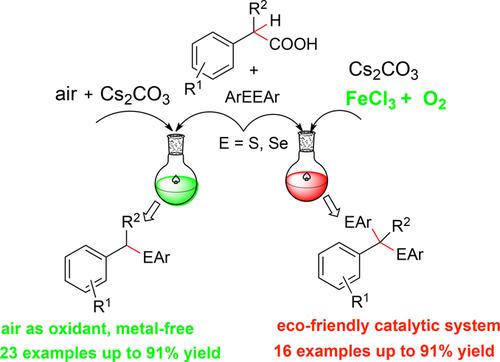
An efficient and selective approach to the synthesis of selenide ethers containing one or two geminal C–Se bonds from readily available diselenides and phenylacetic acids was developed. Compounds containing one C–Se bond were prepared by employing air as the oxidant under metal-free conditions, whereas compounds having two geminal C–Se bonds were formed via the iron(III) chloride/oxygen/cesium carbonate (FeCl3/O2/Cs2CO3) system. Moreover, 1,2-diphenyldisulfane also could be smoothly converted into the corresponding sulfur ether product under the standard reaction conditions.
中文翻译:

通过脱羧偶联反应区域选择性合成硒醚
已开发出一种有效的,选择性的方法,可以从容易获得的二硒化物和苯乙酸合成含一个或两个双键C-Se键的硒醚。通过在无金属条件下将空气用作氧化剂来制备包含一个C-Se键的化合物,而通过氯化铁(III)/氧/碳酸铯(FeCl 3 / O 2)形成具有两个双键C-Se键的化合物。/ Cs 2 CO 3)系统。此外,在标准反应条件下,1,2-二苯基二硫烷也可以平稳地转化为相应的硫醚产物。
更新日期:2017-11-15
中文翻译:

通过脱羧偶联反应区域选择性合成硒醚
已开发出一种有效的,选择性的方法,可以从容易获得的二硒化物和苯乙酸合成含一个或两个双键C-Se键的硒醚。通过在无金属条件下将空气用作氧化剂来制备包含一个C-Se键的化合物,而通过氯化铁(III)/氧/碳酸铯(FeCl 3 / O 2)形成具有两个双键C-Se键的化合物。/ Cs 2 CO 3)系统。此外,在标准反应条件下,1,2-二苯基二硫烷也可以平稳地转化为相应的硫醚产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号