当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of 3β-Hydroxy-7β-kemp-8(9)-en-6-one, a Diterpene Isolated from Higher Termites
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-15 05:37:04 , DOI: 10.1002/anie.201708561 Yuzhou Wang 1 , Anne Jäger 1 , Margit Gruner 1 , Tilo Lübken 1 , Peter Metz 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-15 05:37:04 , DOI: 10.1002/anie.201708561 Yuzhou Wang 1 , Anne Jäger 1 , Margit Gruner 1 , Tilo Lübken 1 , Peter Metz 1
Affiliation
|
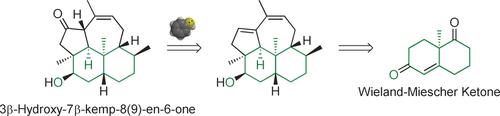
Sulfur helps! The first total synthesis of the title diterpene was accomplished starting from the Wieland–Miescher ketone. A diastereoselective sulfa-Michael addition enabled the generation of the delicate β,γ-unsaturated ketone moiety, while the tetracyclic kempane skeleton was readily constructed through domino metathesis.
中文翻译:

从高白蚁分离出的二萜3β-羟基-7β-kemp-8(9)-en-6-one的对映选择性全合成
硫磺有帮助!从Wieland-Miescher酮开始,完成了标题二萜的第一个全合成。非对映选择性的磺胺基-迈克尔基加成反应可生成精致的β,γ-不饱和酮部分,而四环丙烷骨架可通过多米诺复分解轻松构建。
更新日期:2017-11-15
中文翻译:

从高白蚁分离出的二萜3β-羟基-7β-kemp-8(9)-en-6-one的对映选择性全合成
硫磺有帮助!从Wieland-Miescher酮开始,完成了标题二萜的第一个全合成。非对映选择性的磺胺基-迈克尔基加成反应可生成精致的β,γ-不饱和酮部分,而四环丙烷骨架可通过多米诺复分解轻松构建。











































 京公网安备 11010802027423号
京公网安备 11010802027423号