PLOS Medicine ( IF 10.5 ) Pub Date : 2017-11-14 , DOI: 10.1371/journal.pmed.1002432 Alexander J Szubert 1 , Andrew J Prendergast 1, 2 , Moira J Spyer 1 , Victor Musiime 3, 4 , Philippa Musoke 5 , Mutsa Bwakura-Dangarembizi 6 , Patricia Nahirya-Ntege 7 , Margaret J Thomason 1 , Emmanuel Ndashimye 4 , Immaculate Nkanya 4 , Oscar Senfuma 3 , Boniface Mudenge 8 , Nigel Klein 9 , Diana M Gibb 1 , A Sarah Walker 1 ,
|
Background
Although WHO recommends viral load (VL) monitoring for those on antiretroviral therapy (ART), availability in low-income countries remains limited. We investigated long-term VL and resistance in HIV-infected children managed without real-time VL monitoring.
Methods and findings
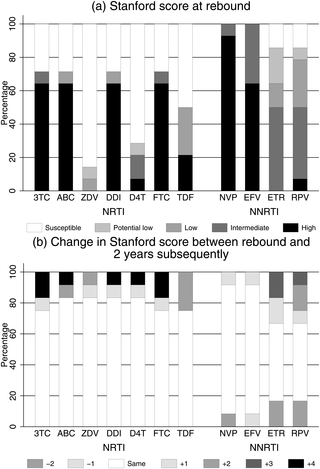
In the ARROW factorial trial, 1,206 children initiating ART in Uganda and Zimbabwe between 15 March 2007 and 18 November 2008, aged a median 6 years old, with median CD4% of 12%, were randomised to monitoring with or without 12-weekly CD4 counts and to receive 2 nucleoside reverse transcriptase inhibitors (2NRTI, mainly abacavir+lamivudine) with a non-nucleoside reverse transcriptase inhibitor (NNRTI) or 3 NRTIs as long-term ART. All children had VL assayed retrospectively after a median of 4 years on ART; those with >1,000 copies/ml were genotyped. Three hundred and sixteen children had VL and genotypes assayed longitudinally (at least every 24 weeks). Overall, 67 (6%) switched to second-line ART and 54 (4%) died. In children randomised to WHO-recommended 2NRTI+NNRTI long-term ART, 308/378 (81%) monitored with CD4 counts versus 297/375 (79%) without had VL <1,000 copies/ml at 4 years (difference = +2.3% [95% CI −3.4% to +8.0%]; P = 0.43), with no evidence of differences in intermediate/high-level resistance to 11 drugs. Among children with longitudinal VLs, only 5% of child-time post–week 24 was spent with persistent low-level viraemia (80–5,000 copies/ml) and 10% with VL rebound ≥5,000 copies/ml. No child resuppressed <80 copies/ml after confirmed VL rebound ≥5,000 copies/ml. A median of 1.0 (IQR 0.0,1.5) additional NRTI mutation accumulated over 2 years’ rebound. Nineteen out of 48 (40%) VLs 1,000–5,000 copies/ml were immediately followed by resuppression <1,000 copies/ml, but only 17/155 (11%) VLs ≥5,000 copies/ml resuppressed (P < 0.0001). Main study limitations are that analyses were exploratory and treatment initiation used 2006 criteria, without pre-ART genotypes.
Conclusions
In this study, children receiving first-line ART in sub-Saharan Africa without real-time VL monitoring had good virological and resistance outcomes over 4 years, regardless of CD4 monitoring strategy. Many children with detectable low-level viraemia spontaneously resuppressed, highlighting the importance of confirming virological failure before switching to second-line therapy. Children experiencing rebound ≥5,000 copies/ml were much less likely to resuppress, but NRTI resistance increased only slowly. These results are relevant to the increasing numbers of HIV-infected children receiving first-line ART in sub-Saharan Africa with limited access to virological monitoring.
Trial registration
ISRCTN Registry, ISRCTN24791884
中文翻译:

乌干达和津巴布韦在没有病毒学监测的情况下接受长期抗逆转录病毒治疗的艾滋病毒感染儿童的病毒学反应和耐药性:随机 ARROW 试验中的观察分析
背景
尽管世卫组织建议对接受抗逆转录病毒治疗(ART)的患者进行病毒载量(VL)监测,但低收入国家的可用性仍然有限。我们调查了没有实时 VL 监测的 HIV 感染儿童的长期 VL 和耐药性。
方法和结果
在 ARROW 析因试验中,2007 年 3 月 15 日至 2008 年 11 月 18 日期间,乌干达和津巴布韦有 1,206 名开始接受 ART 的儿童,年龄中位数为 6 岁,CD4% 中位数为 12%,被随机分为接受或不接受每周 12 周 CD4 计数的监测接受2种核苷逆转录酶抑制剂(2NRTI,主要是阿巴卡韦+拉米夫定)与一种非核苷逆转录酶抑制剂(NNRTI)或3种NRTI作为长期ART。所有儿童在接受 ART 治疗平均 4 年后进行了回顾性 VL 测定;对那些> 1,000 拷贝/毫升的基因进行了基因分型。对 316 名儿童进行了纵向 VL 和基因型检测(至少每 24 周一次)。总体而言,67 例 (6%) 患者改用二线 ART,54 例 (4%) 患者死亡。在随机接受WHO推荐的2NRTI+NNRTI长期ART的儿童中,308/378(81%)接受CD4计数监测,而297/375(79%)没有监测VL<1(P=0.43),没有监测CD4计数。对 11 种药物的中/高水平耐药性差异的证据。在患有纵向 VL 的儿童中,第 24 周后,只有 5% 的儿童时间出现持续低水平病毒血症(80-5,000 拷贝/ml),10% 的 VL 反弹≥5,000 拷贝/ml。没有儿童被重新抑制<80 id=11>P < 0.0001)。主要研究局限性是分析是探索性的,治疗开始时使用 2006 年的标准,没有 ART 前的基因型。
结论
在这项研究中,无论 CD4 监测策略如何,撒哈拉以南非洲地区接受一线 ART 且没有实时 VL 监测的儿童在 4 年内都取得了良好的病毒学和耐药性结果。许多可检测到的低水平病毒血症的儿童会自发地重新抑制,这凸显了在转向二线治疗之前确认病毒学失败的重要性。经历反弹≥5,000拷贝/ml的儿童重新抑制的可能性要小得多,但NRTI耐药性仅缓慢增加。这些结果与撒哈拉以南非洲地区越来越多的艾滋病毒感染儿童接受一线抗逆转录病毒治疗有关,但获得病毒学监测的机会有限。
试用注册
ISRCTN 注册表, ISRCTN24791884










































 京公网安备 11010802027423号
京公网安备 11010802027423号