当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni(I)–Ni(III) vs. Ni(II)–Ni(IV): mechanistic study of Ni-catalyzed alkylation of benzamides with alkyl halides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7qo00850c Yingzi Li 1, 2, 3, 4 , Lufeng Zou 5, 6, 7 , Ruopeng Bai 1, 2, 3, 4 , Yu Lan 1, 2, 3, 4, 8
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7qo00850c Yingzi Li 1, 2, 3, 4 , Lufeng Zou 5, 6, 7 , Ruopeng Bai 1, 2, 3, 4 , Yu Lan 1, 2, 3, 4, 8
Affiliation
|
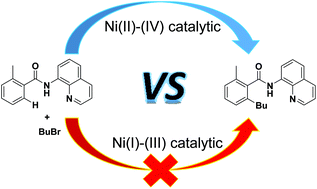
Nickel-catalyzed C–H bond activation has attracted significant attention for the construction of C–C bond frameworks. We report density functional theory investigations into the mechanism of nickel-catalyzed alkylation of benzamides with alkyl halides. Both the Ni(I)–Ni(III) and Ni(II)–Ni(IV) catalytic cycles were considered. The theoretical study indicated that the most feasible mechanism involved a Ni(II)–Ni(IV) catalytic cycle with four main steps: (i) N–H bond activation and (ii) C–H bond activation through the concerted metalation–deprotonation pathway, (iii) oxidative addition of BuBr to give a high-valent Ni(IV) complex, and (iv) C–C reductive elimination to generate the product and the active catalyst. The rate-determining step of the favored pathway is the oxidative addition, leading to the generation of a Ni(IV) intermediate. In addition, the present study casts light on the role of PPh3, which accelerates the cleavage of N–H bond. Frontier molecular orbital theory and natural population analysis were employed to explain the effect of the phosphine ligand on the structure of the Ni complex.
中文翻译:

Ni(I)–Ni(III)vs . Ni(II)–Ni(IV):Ni催化烷基卤化物与苯甲酰胺烷基化的机理研究
镍催化的C–H键活化已引起人们对C–C键构架的关注。我们报告密度泛函理论调查机制的镍与烷基卤化物的苯甲酰胺镍催化的烷基化。同时考虑了Ni(I)–Ni(III)和Ni(II)–Ni(IV)的催化循环。理论研究表明,最可行的机理涉及Ni(II)–Ni(IV)催化循环,该催化循环包括四个主要步骤:(i)N–H键活化和(ii)通过协同金属化-去质子化进行的C–H键活化途径,(iii)BuBr的氧化加成得到高价的Ni(IV)络合物,以及(iv)C–C还原消除反应生成产物和活性催化剂。有利途径的决定速率的步骤是氧化加成反应,导致生成Ni(IV)中间体。另外,本研究阐明了PPh 3的作用,该作用加速了NH键的裂解。前沿分子轨道理论和自然种群分析被用来解释膦配体对镍配合物结构的影响。
更新日期:2017-11-15
中文翻译:

Ni(I)–Ni(III)vs . Ni(II)–Ni(IV):Ni催化烷基卤化物与苯甲酰胺烷基化的机理研究
镍催化的C–H键活化已引起人们对C–C键构架的关注。我们报告密度泛函理论调查机制的镍与烷基卤化物的苯甲酰胺镍催化的烷基化。同时考虑了Ni(I)–Ni(III)和Ni(II)–Ni(IV)的催化循环。理论研究表明,最可行的机理涉及Ni(II)–Ni(IV)催化循环,该催化循环包括四个主要步骤:(i)N–H键活化和(ii)通过协同金属化-去质子化进行的C–H键活化途径,(iii)BuBr的氧化加成得到高价的Ni(IV)络合物,以及(iv)C–C还原消除反应生成产物和活性催化剂。有利途径的决定速率的步骤是氧化加成反应,导致生成Ni(IV)中间体。另外,本研究阐明了PPh 3的作用,该作用加速了NH键的裂解。前沿分子轨道理论和自然种群分析被用来解释膦配体对镍配合物结构的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号