Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood–retinal barrier†
Lab on a Chip ( IF 6.1 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1039/c7lc00795g Jose Yeste 1, 2, 3, 4, 5 , Marta García-Ramírez 3, 6, 7, 8, 9 , Xavi Illa 1, 2, 3, 4, 5 , Anton Guimerà 1, 2, 3, 4, 5 , Cristina Hernández 3, 6, 7, 8, 9 , Rafael Simó 3, 6, 7, 8, 9 , Rosa Villa 1, 2, 3, 4, 5
Lab on a Chip ( IF 6.1 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1039/c7lc00795g Jose Yeste 1, 2, 3, 4, 5 , Marta García-Ramírez 3, 6, 7, 8, 9 , Xavi Illa 1, 2, 3, 4, 5 , Anton Guimerà 1, 2, 3, 4, 5 , Cristina Hernández 3, 6, 7, 8, 9 , Rafael Simó 3, 6, 7, 8, 9 , Rosa Villa 1, 2, 3, 4, 5
Affiliation
|
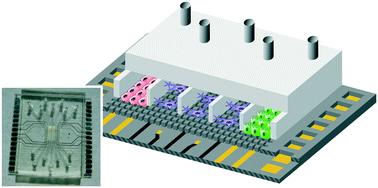
The interconnection of different tissue–tissue interfaces may extend organ-on-chips to a new generation of sophisticated models capable of recapitulating more complex organ-level functions. Single interfaces are largely recreated in organ-on-chips by culturing the cells on opposite sides of a porous membrane that splits a chamber in two or by connecting the cells of two adjacent compartments through microchannels. However, it is difficult to interconnect more than one interface using these approaches. To address this challenge, we present a novel microfluidic device where cells are arranged in parallel compartments and are highly interconnected through a grid of microgrooves, which facilitates paracrine signaling and heterotypic cell–cell contact between multiple tissues. In addition, the device includes electrodes on the substrate for the measurement of transepithelial electrical resistance (TEER). Unlike conventional methods for measuring the TEER where electrodes are on each side of the cell barrier, a method with only electrodes on the substrate has been validated. As a proof-of-concept, we have used the device to mimic the structure of the blood–retinal barrier by co-culturing primary human retinal endothelial cells (HREC), a human neuroblastoma cell line (SH-SY5Y), and a human retinal pigment epithelial cell line (ARPE-19). Cell barrier formations were assessed by a permeability assay, TEER measurements, and ZO-1 expression. These results validate the proposed microfluidic device with microgrooves as a promising in vitro tool for the compartmentalization and monitoring of barrier tissues.
中文翻译:

带有交叉微槽和电生理电极的分隔微流控芯片,用于建模血视网膜屏障†
不同组织间接口的互连可能将片上器官扩展到新一代复杂模型,能够概括更复杂的器官级功能。通过在将腔室一分为二的多孔膜的相对侧上培养细胞,或通过微通道连接两个相邻隔室的细胞,可以在芯片上器官中大量重建单个界面。但是,使用这些方法很难互连多个接口。为了解决这一挑战,我们提出了一种新颖的微流控设备,该设备中的细胞排列在平行的隔室中,并通过微沟槽的网格高度互连,这有助于旁分泌信号传导和多种组织之间的异型细胞间接触。此外,该设备在基板上包括用于测量跨上皮电阻(TEER)的电极。与传统的测量TEER的方法不同,TEER的方法是在电池壁障的每一侧上放置电极,而仅在衬底上放置电极的方法已经过验证。作为概念验证,我们已使用该设备通过共同培养人视网膜原代内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 与传统的测量TEER的方法不同,TEER的方法是在电池壁障的每一侧上放置电极,而仅在衬底上放置电极的方法已经过验证。作为概念验证,我们已使用该设备通过共同培养人视网膜原代内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 与传统的测量TEER的方法不同,TEER的方法是在电池壁障的每一侧上放置电极,而仅在衬底上放置电极的方法已经过验证。作为概念验证,我们已使用该设备通过共同培养人视网膜原代内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 我们已通过共同培养原代人视网膜内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-)来模拟血视网膜屏障的结构19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 我们已通过共同培养原代人视网膜内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-)来模拟血视网膜屏障的结构19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的隔离和监测屏障组织的体外工具。
更新日期:2017-11-14
中文翻译:

带有交叉微槽和电生理电极的分隔微流控芯片,用于建模血视网膜屏障†
不同组织间接口的互连可能将片上器官扩展到新一代复杂模型,能够概括更复杂的器官级功能。通过在将腔室一分为二的多孔膜的相对侧上培养细胞,或通过微通道连接两个相邻隔室的细胞,可以在芯片上器官中大量重建单个界面。但是,使用这些方法很难互连多个接口。为了解决这一挑战,我们提出了一种新颖的微流控设备,该设备中的细胞排列在平行的隔室中,并通过微沟槽的网格高度互连,这有助于旁分泌信号传导和多种组织之间的异型细胞间接触。此外,该设备在基板上包括用于测量跨上皮电阻(TEER)的电极。与传统的测量TEER的方法不同,TEER的方法是在电池壁障的每一侧上放置电极,而仅在衬底上放置电极的方法已经过验证。作为概念验证,我们已使用该设备通过共同培养人视网膜原代内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 与传统的测量TEER的方法不同,TEER的方法是在电池壁障的每一侧上放置电极,而仅在衬底上放置电极的方法已经过验证。作为概念验证,我们已使用该设备通过共同培养人视网膜原代内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 与传统的测量TEER的方法不同,TEER的方法是在电池壁障的每一侧上放置电极,而仅在衬底上放置电极的方法已经过验证。作为概念验证,我们已使用该设备通过共同培养人视网膜原代内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 我们已通过共同培养原代人视网膜内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-)来模拟血视网膜屏障的结构19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的 我们已通过共同培养原代人视网膜内皮细胞(HREC),人神经母细胞瘤细胞系(SH-SY5Y)和人视网膜色素上皮细胞系(ARPE-)来模拟血视网膜屏障的结构19)。通过渗透性测定,TEER测量和ZO-1表达评估细胞屏障形成。这些结果验证了所提出的带有微槽的微流体装置是有前途的隔离和监测屏障组织的体外工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号