当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly selective sp3 C–N bond activation of tertiary anilines modulated by steric and thermodynamic factors
Green Chemistry ( IF 9.8 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7gc02775c Xiaodong Jia 1, 2, 3, 4 , Pengfei Li 4, 5, 6, 7 , Yu Shao 2, 3, 4, 8 , Yu Yuan 1, 2, 3, 4 , Honghe Ji 4, 5, 6, 7 , Wentao Hou 4, 5, 6, 7 , Xiaofei Liu 4, 5, 6, 7 , Xuewen Zhang 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7gc02775c Xiaodong Jia 1, 2, 3, 4 , Pengfei Li 4, 5, 6, 7 , Yu Shao 2, 3, 4, 8 , Yu Yuan 1, 2, 3, 4 , Honghe Ji 4, 5, 6, 7 , Wentao Hou 4, 5, 6, 7 , Xiaofei Liu 4, 5, 6, 7 , Xuewen Zhang 1, 2, 3, 4
Affiliation
|
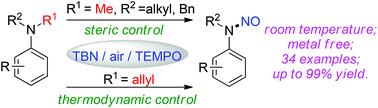
A highly selective sp3 C–N cleavage of tertiary anilines was achieved using the TBN/TEMPO catalyst system. When N,N-diaklylanilines (alkyl, benzyl) were employed, the N–CH3 bond was selectively cleaved via radical C–H activation. Moreover, when the allyl group was installed, totally reverse selectivity was observed. It is worth noting that the solvent effect is also crucial to obtain high reaction efficiency and selectivity.
中文翻译:

空间和热力学因素调节的高选择性sp 3 C–N键活化叔苯胺
使用TBN / TEMPO催化剂系统可实现对叔苯胺的高选择性sp 3 C–N裂解。当使用N,N-二烯丙基苯胺(烷基,苄基)时,N–CH 3键通过C–H自由基活化被选择性裂解。此外,当安装烯丙基时,观察到完全相反的选择性。值得注意的是,溶剂效应对于获得高反应效率和选择性也至关重要。
更新日期:2017-11-15
中文翻译:

空间和热力学因素调节的高选择性sp 3 C–N键活化叔苯胺
使用TBN / TEMPO催化剂系统可实现对叔苯胺的高选择性sp 3 C–N裂解。当使用N,N-二烯丙基苯胺(烷基,苄基)时,N–CH 3键通过C–H自由基活化被选择性裂解。此外,当安装烯丙基时,观察到完全相反的选择性。值得注意的是,溶剂效应对于获得高反应效率和选择性也至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号