当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalyzed Anionic Ring-Opening Polymerizations of N-Sulfonyl Aziridines with Organic Superbases
ACS Macro Letters ( IF 5.1 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acsmacrolett.7b00775 Xin Wang 1 , Yaya Liu 1 , Zhenjiang Li 1 , Haixin Wang 1 , Hailemariam Gebru 1 , Siming Chen 1 , Hui Zhu 1 , Fulan Wei 1 , Kai Guo 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acsmacrolett.7b00775 Xin Wang 1 , Yaya Liu 1 , Zhenjiang Li 1 , Haixin Wang 1 , Hailemariam Gebru 1 , Siming Chen 1 , Hui Zhu 1 , Fulan Wei 1 , Kai Guo 1
Affiliation
|
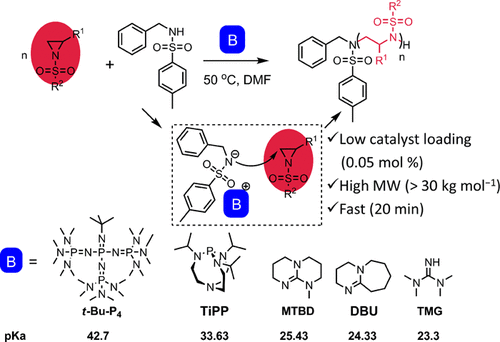
The anionic ring-opening polymerizations (AROPs) of N-sulfonyl aziridines, in the presence of organic superbases including phosphazene (t-Bu-P4), Verkade’s base (P(i-PrNCH2CH2)3N, TiPP), DBU, MTBD, and N,N,N′,N′-tetramethylguanidine (TMG), using N-benzyl-p-toluenesulfonamide (BnN(H)Ts) as initiator were explored to produce metal-free poly(sulfonylaziridine)s. Among the superbases used, the catalytic activity was found directly proportional to their basicity. Remarkably, t-Bu-P4 and TiPP gave a living/controlled AROP of 2-methyl-N-tosylaziridine (TsMAz), where t-Bu-P4 performed better, affording the metal-free and well-defined poly(sulfonylaziridine)s with high molar masses (Mn(SEC) > 30 kg mol–1) and low dispersities (Đ < 1.10) in 3.5 h. For the less reactive monomers of 2-methyl-N-ethylsulfonyl aziridine (EsMAz) and 2-phenyl-N-tosylaziridine (TsPhAz), t-Bu-P4 showed the same excellent catalytic efficiency (30 equiv, conv. > 95%, 5 h). The organocatalyzed AROP allowed the use of lower catalyst (t-Bu-P4) loading than the amount of initiator (BnN(H)Ts), but the propagating polymer chains were as many as the number of equivalents of the introduced initiators, which could lower the loading of catalyst used to amounts as low as 0.05 mol %.
中文翻译:

N-磺酰基氮丙啶与有机超碱的有机催化阴离子开环聚合
N-磺酰基氮丙啶的阴离子开环聚合 (AROPs) ,在有机超碱包括磷腈 ( t -Bu-P 4 )、Verkade 碱 (P( i -PrNCH 2 CH 2 ) 3 N, TiPP) 的存在下, DBU、MTBD 和N , N , N ', N '-四甲基胍 (TMG) 以N-苄基-对甲苯磺酰胺 (BnN(H)Ts) 作为引发剂进行了探索以生产不含金属的聚(磺酰基氮丙啶)。在使用的超碱中,发现催化活性与它们的碱度成正比。值得注意的是,t -Bu-P4和TiPP产生2-甲基-N-甲苯磺酰基氮丙啶(TsMAz)的活性/可控AROP,其中t -Bu-P 4表现更好,提供具有高摩尔质量的无金属和明确定义的聚(磺酰基氮丙啶)(Mn (SEC) > 30 kg mol –1 )和低分散性 ( Đ < 1.10) 在 3.5 小时内。对于反应性较低的 2-甲基-N-乙基磺酰基氮丙啶 (EsMAz) 和 2-苯基-N-甲苯磺酰基氮丙啶 (TsPhAz) 单体,t -Bu -P 4表现出同样优异的催化效率(30 equiv,转化率 > 95% , 5 小时)。有机催化的 AROP 允许使用较低的催化剂 ( t -Bu-P4 ) 负载量比引发剂 (BnN(H)Ts) 的量高,但增长的聚合物链与引入的引发剂的当量数一样多,这可以将所用催化剂的负载量降低至 0.05 mol % .
更新日期:2017-11-14
中文翻译:

N-磺酰基氮丙啶与有机超碱的有机催化阴离子开环聚合
N-磺酰基氮丙啶的阴离子开环聚合 (AROPs) ,在有机超碱包括磷腈 ( t -Bu-P 4 )、Verkade 碱 (P( i -PrNCH 2 CH 2 ) 3 N, TiPP) 的存在下, DBU、MTBD 和N , N , N ', N '-四甲基胍 (TMG) 以N-苄基-对甲苯磺酰胺 (BnN(H)Ts) 作为引发剂进行了探索以生产不含金属的聚(磺酰基氮丙啶)。在使用的超碱中,发现催化活性与它们的碱度成正比。值得注意的是,t -Bu-P4和TiPP产生2-甲基-N-甲苯磺酰基氮丙啶(TsMAz)的活性/可控AROP,其中t -Bu-P 4表现更好,提供具有高摩尔质量的无金属和明确定义的聚(磺酰基氮丙啶)(Mn (SEC) > 30 kg mol –1 )和低分散性 ( Đ < 1.10) 在 3.5 小时内。对于反应性较低的 2-甲基-N-乙基磺酰基氮丙啶 (EsMAz) 和 2-苯基-N-甲苯磺酰基氮丙啶 (TsPhAz) 单体,t -Bu -P 4表现出同样优异的催化效率(30 equiv,转化率 > 95% , 5 小时)。有机催化的 AROP 允许使用较低的催化剂 ( t -Bu-P4 ) 负载量比引发剂 (BnN(H)Ts) 的量高,但增长的聚合物链与引入的引发剂的当量数一样多,这可以将所用催化剂的负载量降低至 0.05 mol % .











































 京公网安备 11010802027423号
京公网安备 11010802027423号