当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Parameters Influencing Hydrocarbon Selectivity during the Electrochemical Conversion of CO2
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acscatal.7b02373 Joshua T. Billy 1 , Anne C. Co 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acscatal.7b02373 Joshua T. Billy 1 , Anne C. Co 1
Affiliation
|
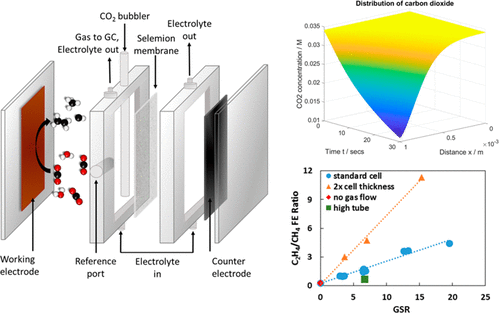
The selectivity of the products formed from the electrochemical conversion of CO2 can be controllably altered by changing the experimental conditions while the catalyst surface is kept the same. Herein, we demonstrate the importance of controlling electrolysis conditions and reporting detailed experimental parameters, such that catalyst activity can be compared objectively, which will aid in our understanding of the complex CO2-reaction processes reported across various research groups. In this work, we report that selectivity toward C2H4 and CO is favored when the kinetics of CO2 equilibration is made faster. Also, local surface pH can be altered by manipulating either buffer capacity or the Nernst-layer thickness; a more acidic surface favors the formation of CH4 and HCOOH on electropolished Cu surfaces, whereas a more basic surface favors C2H4 formation.
中文翻译:

影响CO 2电化学转化过程中烃选择性的实验参数
在保持催化剂表面不变的情况下,通过改变实验条件,可控制地改变由CO 2的电化学转化形成的产物的选择性。在本文中,我们证明了控制电解条件和报告详细的实验参数的重要性,以便可以客观地比较催化剂的活性,这将有助于我们理解各个研究小组报告的复杂的CO 2反应过程。在这项工作中,我们报告说,当CO 2动力学时,对C 2 H 4和CO的选择性有利。平衡变得更快。同样,可以通过控制缓冲容量或能斯特层的厚度来改变局部表面的pH值。酸性更高的表面有利于在电抛光的铜表面上形成CH 4和HCOOH,而碱性更高的表面则有利于C 2 H 4的形成。
更新日期:2017-11-15
中文翻译:

影响CO 2电化学转化过程中烃选择性的实验参数
在保持催化剂表面不变的情况下,通过改变实验条件,可控制地改变由CO 2的电化学转化形成的产物的选择性。在本文中,我们证明了控制电解条件和报告详细的实验参数的重要性,以便可以客观地比较催化剂的活性,这将有助于我们理解各个研究小组报告的复杂的CO 2反应过程。在这项工作中,我们报告说,当CO 2动力学时,对C 2 H 4和CO的选择性有利。平衡变得更快。同样,可以通过控制缓冲容量或能斯特层的厚度来改变局部表面的pH值。酸性更高的表面有利于在电抛光的铜表面上形成CH 4和HCOOH,而碱性更高的表面则有利于C 2 H 4的形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号