Synthesis ( IF 2.2 ) Pub Date : 2017-11-13 , DOI: 10.1055/s-0036-1590956 Katsunori Tanaka 1, 2, 3 , Katsumasa Fujiki 1
|
Abstract
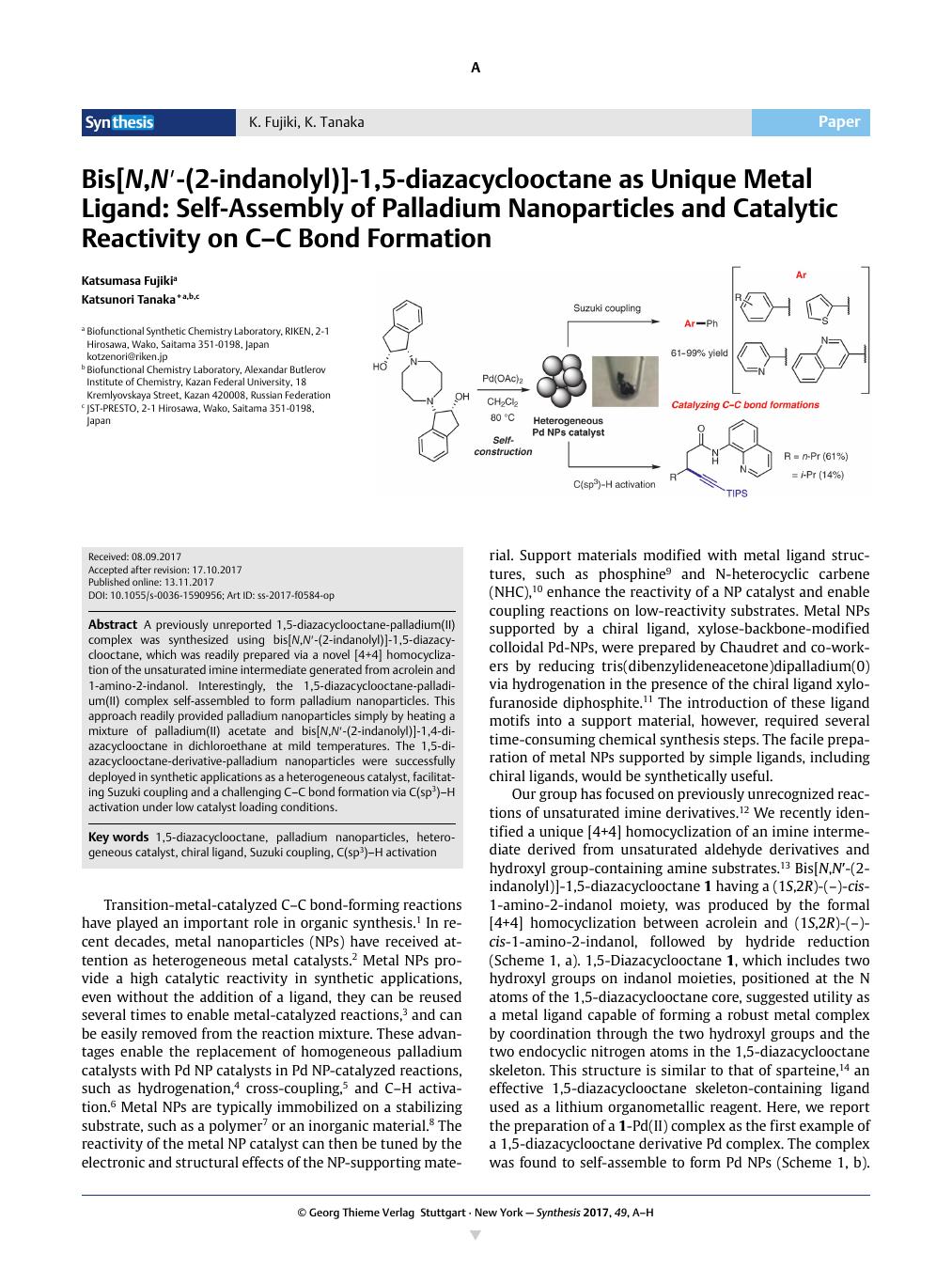
A previously unreported 1,5-diazacyclooctane-palladium(II) complex was synthesized using bis[N,N′-(2-indanolyl)]-1,5-diazacyclooctane, which was readily prepared via a novel [4+4] homocyclization of the unsaturated imine intermediate generated from acrolein and 1-amino-2-indanol. Interestingly, the 1,5-diazacyclooctane-palladium(II) complex self-assembled to form palladium nanoparticles. This approach readily provided palladium nanoparticles simply by heating a mixture of palladium(II) acetate and bis[N,N′-(2-indanolyl)]-1,4-diazacyclooctane in dichloroethane at mild temperatures. The 1,5-diazacyclooctane-derivative-palladium nanoparticles were successfully deployed in synthetic applications as a heterogeneous catalyst, facilitating Suzuki coupling and a challenging C–C bond formation via C(sp3)–H activation under low catalyst loading conditions.
A previously unreported 1,5-diazacyclooctane-palladium(II) complex was synthesized using bis[N,N′-(2-indanolyl)]-1,5-diazacyclooctane, which was readily prepared via a novel [4+4] homocyclization of the unsaturated imine intermediate generated from acrolein and 1-amino-2-indanol. Interestingly, the 1,5-diazacyclooctane-palladium(II) complex self-assembled to form palladium nanoparticles. This approach readily provided palladium nanoparticles simply by heating a mixture of palladium(II) acetate and bis[N,N′-(2-indanolyl)]-1,4-diazacyclooctane in dichloroethane at mild temperatures. The 1,5-diazacyclooctane-derivative-palladium nanoparticles were successfully deployed in synthetic applications as a heterogeneous catalyst, facilitating Suzuki coupling and a challenging C–C bond formation via C(sp3)–H activation under low catalyst loading conditions.
中文翻译:

双[N,N'-(2-茚满基)]-1,5-二氮杂环辛烷作为独特的金属配体:钯纳米粒子的自组装和在C–C键形成上的催化反应性
摘要
使用双[ N,N '-(2-茚满基)]-1,5-二氮杂环辛烷合成了先前未报道的1,5-二氮杂环辛烷-钯(II)配合物,该化合物可通过新型[4 + 4]均环化轻松制备由丙烯醛和1-氨基-2-茚满醇生成的不饱和亚胺中间体。有趣的是,1,5-二氮杂环辛烷-钯(II)络合物自组装形成钯纳米颗粒。通过加热乙酸钯(II)和双[ N,N在温和的温度下,在二氯乙烷中的'-(2-茚满基)]-1,4-二氮杂环辛烷。1,5-二氮杂环辛烷衍生物钯纳米颗粒已成功地在合成应用中用作非均相催化剂,促进了铃木偶联和在低催化剂负载条件下通过C(sp 3)-H活化形成具有挑战性的C–C键。
使用双[ N,N '-(2-茚满基)]-1,5-二氮杂环辛烷合成了先前未报道的1,5-二氮杂环辛烷-钯(II)配合物,该化合物可通过新型[4 + 4]均环化轻松制备由丙烯醛和1-氨基-2-茚满醇生成的不饱和亚胺中间体。有趣的是,1,5-二氮杂环辛烷-钯(II)络合物自组装形成钯纳米颗粒。通过加热乙酸钯(II)和双[ N,N在温和的温度下,在二氯乙烷中的'-(2-茚满基)]-1,4-二氮杂环辛烷。1,5-二氮杂环辛烷衍生物钯纳米颗粒已成功地在合成应用中用作非均相催化剂,促进了铃木偶联和在低催化剂负载条件下通过C(sp 3)-H活化形成具有挑战性的C–C键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号