当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembled Polypeptide Nanogels with Enzymatically Transformable Surface as a Small Interfering RNA Delivery Platform
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.biomac.7b00937 Tomoki Nishimura 1, 2 , Akina Yamada 1 , Kaori Umezaki 2 , Shin-ichi Sawada 1, 2 , Sada-atsu Mukai 1, 2 , Yoshihiro Sasaki 1 , Kazunari Akiyoshi 1, 2
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.biomac.7b00937 Tomoki Nishimura 1, 2 , Akina Yamada 1 , Kaori Umezaki 2 , Shin-ichi Sawada 1, 2 , Sada-atsu Mukai 1, 2 , Yoshihiro Sasaki 1 , Kazunari Akiyoshi 1, 2
Affiliation
|
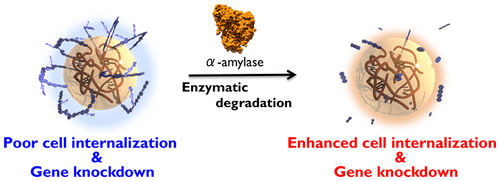
Nanometer-size gel particles, or nanogels, have potential for delivering therapeutic macromolecules. A cationic surface promotes cellular internalization of nanogels, but undesired electrostatic interactions, such as with blood components, cause instability and toxicities. Poly(ethylene glycol) coating has been used to shield charges, but this decreases delivery efficiency. Technical difficulties in synthesis and controlling molecular weights make it unfeasible to, instead, coat with biodegradable polymers. Our proposed solution is cationized nanogels enzymatically functionalized with branched polysaccharide chains, forming a shell to shield charges and increase stability. Biodegradation of the polysaccharides by an endogenous enzyme would then expose the cationic charges, allowing cellular internalization and cargo delivery. We tested this concept, preparing maltopentaose functionalized cholesteryl poly(l-lysine) nanogel and using tandem enzymatic polymerization with glycogen phosphorylase and glycogen branching enzyme, to add branched amylose moieties, forming a CbAmyPL nanogel. We characterized CbAmyPL nanogels and investigated their suitability as small interfering RNA (siRNA) carriers in murine renal carcinoma (Renca) cells. The nanogels had neutral ζ potential values that became positive after degradation by α-amylase. Foster resonance energy transfer demonstrated that the nanogels formed stable complexes with siRNA, even in the presence of bovine serum albumin and after α-amylase exposure. The nanogels, with or without α-amylase, were not cytotoxic. Complexes of CbAmyPL with siRNA against vascular endothelial growth factor (VEGF), when incubated alone with Renca cells decreased VEGF mRNA levels by only 20%. With α-amylase added, however, VEGF mRNA knockdown by the siRNA/nanogels complexes was 50%. Our findings strongly supported the hypothesis that enzyme-responsive nanogels are promising as a therapeutic siRNA delivery platform.
中文翻译:

自组装的多肽纳米凝胶,具有可酶转化的表面,作为小的干扰RNA传递平台。
纳米大小的凝胶颗粒或纳米凝胶具有释放治疗性大分子的潜力。阳离子表面促进纳米凝胶的细胞内在化,但是不希望有的静电相互作用(例如与血液成分的相互作用)会导致不稳定和毒性。聚乙二醇涂层已被用来屏蔽电荷,但这降低了输送效率。合成和控制分子量方面的技术困难使得用生物可降解聚合物包衣是不可行的。我们提出的解决方案是用支化的多糖链酶促功能化的阳离子化纳米凝胶,形成可屏蔽电荷并增加稳定性的壳。内源性酶对多糖的生物降解作用随后会暴露出阳离子电荷,从而使细胞内化并运送货物。升-赖氨酸)纳米凝胶,并使用糖原磷酸化酶和糖原支化酶进行串联酶促聚合反应,以添加支链直链部分,形成CbAmyPL纳米凝胶。我们表征了CbAmyPL纳米凝胶,并研究了它们在鼠肾癌(Renca)细胞中作为小型干扰RNA(siRNA)载体的适用性。纳米凝胶具有中性的ζ电位值,该值在被α-淀粉酶降解后变为正值。福斯特共振能量转移表明,即使在牛血清白蛋白存在下和暴露于α-淀粉酶之后,纳米凝胶也能与siRNA形成稳定的复合物。具有或不具有α-淀粉酶的纳米凝胶均无细胞毒性。当与Renca细胞单独孵育时,CbAmyPL与siRNA对抗血管内皮生长因子(VEGF)的复合物仅会使VEGF mRNA水平降低20%。但是,添加α-淀粉酶后,siRNA / nanogels复合物对VEGF mRNA的抑制作用为50%。我们的发现强烈支持酶反应性纳米凝胶有望作为治疗性siRNA传递平台的假设。
更新日期:2017-11-14
中文翻译:

自组装的多肽纳米凝胶,具有可酶转化的表面,作为小的干扰RNA传递平台。
纳米大小的凝胶颗粒或纳米凝胶具有释放治疗性大分子的潜力。阳离子表面促进纳米凝胶的细胞内在化,但是不希望有的静电相互作用(例如与血液成分的相互作用)会导致不稳定和毒性。聚乙二醇涂层已被用来屏蔽电荷,但这降低了输送效率。合成和控制分子量方面的技术困难使得用生物可降解聚合物包衣是不可行的。我们提出的解决方案是用支化的多糖链酶促功能化的阳离子化纳米凝胶,形成可屏蔽电荷并增加稳定性的壳。内源性酶对多糖的生物降解作用随后会暴露出阳离子电荷,从而使细胞内化并运送货物。升-赖氨酸)纳米凝胶,并使用糖原磷酸化酶和糖原支化酶进行串联酶促聚合反应,以添加支链直链部分,形成CbAmyPL纳米凝胶。我们表征了CbAmyPL纳米凝胶,并研究了它们在鼠肾癌(Renca)细胞中作为小型干扰RNA(siRNA)载体的适用性。纳米凝胶具有中性的ζ电位值,该值在被α-淀粉酶降解后变为正值。福斯特共振能量转移表明,即使在牛血清白蛋白存在下和暴露于α-淀粉酶之后,纳米凝胶也能与siRNA形成稳定的复合物。具有或不具有α-淀粉酶的纳米凝胶均无细胞毒性。当与Renca细胞单独孵育时,CbAmyPL与siRNA对抗血管内皮生长因子(VEGF)的复合物仅会使VEGF mRNA水平降低20%。但是,添加α-淀粉酶后,siRNA / nanogels复合物对VEGF mRNA的抑制作用为50%。我们的发现强烈支持酶反应性纳米凝胶有望作为治疗性siRNA传递平台的假设。









































 京公网安备 11010802027423号
京公网安备 11010802027423号