当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphate Promotes the Recovery of Mycobacterium tuberculosis β-Lactamase from Clavulanic Acid Inhibition
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00556 Wouter Elings 1 , Raffaella Tassoni 1 , Steven A. van der Schoot 1 , Wendy Luu 1 , Josef P. Kynast 1 , Lin Dai 1 , Anneloes J. Blok 1 , Monika Timmer 1 , Bogdan I. Florea 1 , Navraj S. Pannu 1 , Marcellus Ubbink 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00556 Wouter Elings 1 , Raffaella Tassoni 1 , Steven A. van der Schoot 1 , Wendy Luu 1 , Josef P. Kynast 1 , Lin Dai 1 , Anneloes J. Blok 1 , Monika Timmer 1 , Bogdan I. Florea 1 , Navraj S. Pannu 1 , Marcellus Ubbink 1
Affiliation
|
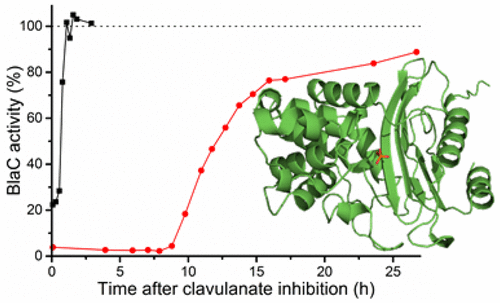
The rise of multi- and even totally antibiotic resistant forms of Mycobacterium tuberculosis underlines the need for new antibiotics. The pathogen is resistant to β-lactam compounds due to its native serine β-lactamase, BlaC. This resistance can be circumvented by administration of a β-lactamase inhibitor. We studied the interaction between BlaC and the inhibitor clavulanic acid. Our data show hydrolysis of clavulanic acid and recovery of BlaC activity upon prolonged incubation. The rate of clavulanic acid hydrolysis is much higher in the presence of phosphate ions. A specific binding site for phosphate is identified in the active site pocket, both in the crystalline state and in solution. NMR spectroscopy experiments show that phosphate binds to this site with a dissociation constant of 30 mM in the free enzyme. We conclude that inhibition of BlaC by clavulanic acid is reversible and that phosphate ions can promote the hydrolysis of the inhibitor.
中文翻译:

磷酸盐促进克拉维酸抑制作用恢复结核分枝杆菌β-内酰胺酶
结核分枝杆菌多耐药甚至全耐药形式的兴起强调了对新抗生素的需求。由于其天然的丝氨酸β-内酰胺酶BlaC,病原体对β-内酰胺化合物具有抗性。可以通过施用β-内酰胺酶抑制剂来规避这种抗药性。我们研究了BlaC和抑制剂克拉维酸之间的相互作用。我们的数据显示,在长时间孵育后,棒酸的水解和BlaC活性的恢复。在磷酸根离子存在下,棒酸的水解速率要高得多。在晶体和溶液中的活性位点囊中都鉴定出了磷酸盐的特异性结合位点。NMR光谱实验表明,磷酸盐在游离酶中以30 mM的解离常数与该位点结合。
更新日期:2017-11-14
中文翻译:

磷酸盐促进克拉维酸抑制作用恢复结核分枝杆菌β-内酰胺酶
结核分枝杆菌多耐药甚至全耐药形式的兴起强调了对新抗生素的需求。由于其天然的丝氨酸β-内酰胺酶BlaC,病原体对β-内酰胺化合物具有抗性。可以通过施用β-内酰胺酶抑制剂来规避这种抗药性。我们研究了BlaC和抑制剂克拉维酸之间的相互作用。我们的数据显示,在长时间孵育后,棒酸的水解和BlaC活性的恢复。在磷酸根离子存在下,棒酸的水解速率要高得多。在晶体和溶液中的活性位点囊中都鉴定出了磷酸盐的特异性结合位点。NMR光谱实验表明,磷酸盐在游离酶中以30 mM的解离常数与该位点结合。










































 京公网安备 11010802027423号
京公网安备 11010802027423号