当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Five Protein Engineering Strategies for Stabilizing an α/β-Hydrolase
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00571 Bryan J. Jones 1 , Huey Yee Lim 1 , Jun Huang 1, 2 , Romas J. Kazlauskas 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00571 Bryan J. Jones 1 , Huey Yee Lim 1 , Jun Huang 1, 2 , Romas J. Kazlauskas 1
Affiliation
|
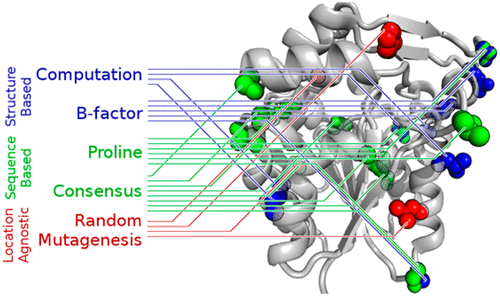
A review of the previous stabilization of α/β-hydrolase fold enzymes revealed many different strategies, but no comparison of strategies on the same enzyme. For this reason, we compared five strategies to identify stabilizing mutations in a model α/β-hydrolase fold enzyme, salicylic acid binding protein 2, to reversible denaturation by urea and to irreversible denaturation by heat. The five strategies included one location agnostic approach (random mutagenesis using error-prone polymerase chain reaction), two structure-based approaches [computational design (Rosetta, FoldX) and mutation of flexible regions], and two sequence-based approaches (addition of proline at locations where a more stable homologue has proline and mutation to consensus). All strategies identified stabilizing mutations, but the best balance of success rate, degree of stabilization, and ease of implementation was mutation to consensus. A web-based automated program that predicts substitutions needed to mutate to consensus is available at http://kazlab.umn.edu.
中文翻译:

稳定α/β-水合酶的五种蛋白质工程策略的比较
对先前的α/β-水解酶折叠酶稳定性的综述揭示了许多不同的策略,但是没有对相同酶的策略进行比较。因此,我们比较了五种策略来确定模型α/β-水解酶折叠酶,水杨酸结合蛋白2中的稳定突变,尿素可逆变性和热不可逆变性。这五种策略包括一种位置不可知方法(使用易于出错的聚合酶链反应进行随机诱变),两种基于结构的方法(计算设计(Rosetta,FoldX)和柔性区域突变),以及两种基于序列的方法(添加脯氨酸)在更稳定的同源物具有脯氨酸且突变为共识的位置)。所有策略都确定了稳定的突变,但成功率的最佳平衡是 稳定程度和易于实施性已转变为共识。可以使用基于网络的自动化程序来预测突变以达成共识所需的替代方案,网址为http://kazlab.umn.edu。
更新日期:2017-11-14
中文翻译:

稳定α/β-水合酶的五种蛋白质工程策略的比较
对先前的α/β-水解酶折叠酶稳定性的综述揭示了许多不同的策略,但是没有对相同酶的策略进行比较。因此,我们比较了五种策略来确定模型α/β-水解酶折叠酶,水杨酸结合蛋白2中的稳定突变,尿素可逆变性和热不可逆变性。这五种策略包括一种位置不可知方法(使用易于出错的聚合酶链反应进行随机诱变),两种基于结构的方法(计算设计(Rosetta,FoldX)和柔性区域突变),以及两种基于序列的方法(添加脯氨酸)在更稳定的同源物具有脯氨酸且突变为共识的位置)。所有策略都确定了稳定的突变,但成功率的最佳平衡是 稳定程度和易于实施性已转变为共识。可以使用基于网络的自动化程序来预测突变以达成共识所需的替代方案,网址为http://kazlab.umn.edu。











































 京公网安备 11010802027423号
京公网安备 11010802027423号