当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed 1,2-Aminoarylation of Oxime Ester-Tethered Alkenes with Boronic Acids
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acscatal.7b03432 Hai-Bin Yang 1 , Stalin R. Pathipati 1 , Nicklas Selander 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acscatal.7b03432 Hai-Bin Yang 1 , Stalin R. Pathipati 1 , Nicklas Selander 1
Affiliation
|
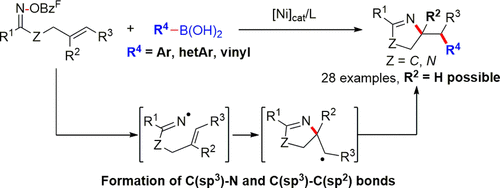
A nickel-catalyzed 1,2-aminoarylation of oxime-ester-tethered alkenes with boronic acids was developed. A variety of pyrroline derivatives were synthesized in good yields via the successive formation of C(sp3)–N and C(sp3)–C(sp2) bonds. For cyclobutanone-derived oxime esters, the reaction provided aliphatic nitriles incorporating an aromatic group in the γ-position. A mechanism involving iminyl radical and carbon-centered radical intermediates was proposed.
中文翻译:

肟催化的硼酸酯对镍催化的肟酯系烯烃的1,2-氨基芳基化
开发了镍催化的肟酯系链烯烃与硼酸的镍催化1,2-氨基芳基化反应。通过连续形成C(sp 3)–N和C(sp 3)–C(sp 2)键,可以合成高产率的各种吡咯啉衍生物。对于环丁酮衍生的肟酯,该反应提供了在γ位上结合有芳族基团的脂族腈。提出了涉及亚胺基和以碳为中心的自由基中间体的机理。
更新日期:2017-11-14
中文翻译:

肟催化的硼酸酯对镍催化的肟酯系烯烃的1,2-氨基芳基化
开发了镍催化的肟酯系链烯烃与硼酸的镍催化1,2-氨基芳基化反应。通过连续形成C(sp 3)–N和C(sp 3)–C(sp 2)键,可以合成高产率的各种吡咯啉衍生物。对于环丁酮衍生的肟酯,该反应提供了在γ位上结合有芳族基团的脂族腈。提出了涉及亚胺基和以碳为中心的自由基中间体的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号