当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic-Enabled Intracellular Delivery of Membrane Impermeable Inhibitors to Study Target Engagement in Human Primary Cells
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acschembio.7b00683 Jing Li 1 , Bu Wang 2 , Brian M. Juba 3 , Michael Vazquez 1 , Steve W. Kortum 4 , Betsy S. Pierce 4 , Michael Pacheco 4 , Lee Roberts 1 , Joseph W. Strohbach 1 , Lyn H. Jones 1 , Erik Hett 1 , Atli Thorarensen 1 , Jean-Baptiste Telliez 3 , Armon Sharei 2 , Mark Bunnage 1 , Jonathan Brian Gilbert 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acschembio.7b00683 Jing Li 1 , Bu Wang 2 , Brian M. Juba 3 , Michael Vazquez 1 , Steve W. Kortum 4 , Betsy S. Pierce 4 , Michael Pacheco 4 , Lee Roberts 1 , Joseph W. Strohbach 1 , Lyn H. Jones 1 , Erik Hett 1 , Atli Thorarensen 1 , Jean-Baptiste Telliez 3 , Armon Sharei 2 , Mark Bunnage 1 , Jonathan Brian Gilbert 2
Affiliation
|
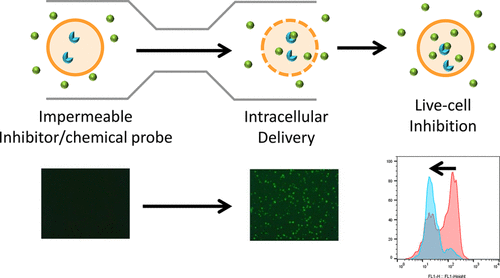
Biochemical screening is a major source of lead generation for novel targets. However, during the process of small molecule lead optimization, compounds with excellent biochemical activity may show poor cellular potency, making structure–activity relationships difficult to decipher. This may be due to low membrane permeability of the molecule, resulting in insufficient intracellular drug concentration. The Cell Squeeze platform increases permeability regardless of compound structure by mechanically disrupting the membrane, which can overcome permeability limitations and bridge the gap between biochemical and cellular studies. In this study, we show that poorly permeable Janus kinase (JAK) inhibitors are delivered into primary cells using Cell Squeeze, inhibiting up to 90% of the JAK pathway, while incubation of JAK inhibitors with or without electroporation had no significant effect. We believe this robust intracellular delivery approach could enable more effective lead optimization and deepen our understanding of target engagement by small molecules and functional probes.
中文翻译:

膜不透性抑制剂的微流控细胞内传递,以研究人类原代细胞中的靶标参与。
生化筛选是新靶标产生铅的主要来源。但是,在小分子先导物优化过程中,具有出色生化活性的化合物可能显示出较差的细胞效力,从而使结构-活性关系难以解读。这可能是由于分子的低膜渗透性,导致细胞内药物浓度不足。Cell Squeeze平台可通过机械破坏膜来增加通透性,无论化合物结构如何,均可克服通透性限制并弥合生化研究与细胞研究之间的差距。在这项研究中,我们证明了使用Cell Squeeze将渗透性差的Janus激酶(JAK)抑制剂递送到原代细胞中,可抑制多达90%的JAK途径,在有或没有电穿孔的情况下孵育JAK抑制剂均无明显效果。我们相信这种强大的细胞内递送方法可以实现更有效的先导优化,并加深我们对小分子和功能探针对靶标结合的了解。
更新日期:2017-11-14
中文翻译:

膜不透性抑制剂的微流控细胞内传递,以研究人类原代细胞中的靶标参与。
生化筛选是新靶标产生铅的主要来源。但是,在小分子先导物优化过程中,具有出色生化活性的化合物可能显示出较差的细胞效力,从而使结构-活性关系难以解读。这可能是由于分子的低膜渗透性,导致细胞内药物浓度不足。Cell Squeeze平台可通过机械破坏膜来增加通透性,无论化合物结构如何,均可克服通透性限制并弥合生化研究与细胞研究之间的差距。在这项研究中,我们证明了使用Cell Squeeze将渗透性差的Janus激酶(JAK)抑制剂递送到原代细胞中,可抑制多达90%的JAK途径,在有或没有电穿孔的情况下孵育JAK抑制剂均无明显效果。我们相信这种强大的细胞内递送方法可以实现更有效的先导优化,并加深我们对小分子和功能探针对靶标结合的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号