当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification and Characterization of Dual Inhibitors of the USP25/28 Deubiquitinating Enzyme Subfamily
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acschembio.7b00334 Jonathan D. Wrigley 1 , Gerald Gavory 2 , Iain Simpson 3 , Marian Preston 1 , Helen Plant 1 , Jenna Bradley 1 , Anne U. Goeppert 1 , Ewelina Rozycka 2 , Gareth Davies 1 , Jarrod Walsh 1 , Andrea Valentine 2 , Keeva McClelland 2 , Krzysztofa Ewa Odrzywol 2 , Jonathan Renshaw 1 , Joanna Boros 1 , Jonathan Tart 1 , Lindsey Leach 1 , Thorsten Nowak 3 , Richard A. Ward 3 , Timothy Harrison 2 , David M. Andrews 3
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acschembio.7b00334 Jonathan D. Wrigley 1 , Gerald Gavory 2 , Iain Simpson 3 , Marian Preston 1 , Helen Plant 1 , Jenna Bradley 1 , Anne U. Goeppert 1 , Ewelina Rozycka 2 , Gareth Davies 1 , Jarrod Walsh 1 , Andrea Valentine 2 , Keeva McClelland 2 , Krzysztofa Ewa Odrzywol 2 , Jonathan Renshaw 1 , Joanna Boros 1 , Jonathan Tart 1 , Lindsey Leach 1 , Thorsten Nowak 3 , Richard A. Ward 3 , Timothy Harrison 2 , David M. Andrews 3
Affiliation
|
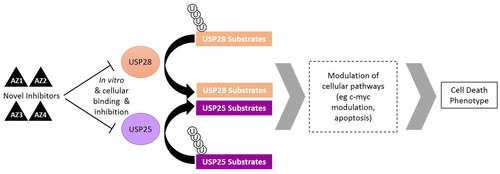
The ubiquitin proteasome system is widely postulated to be a new and important field of drug discovery for the future, with the ubiquitin specific proteases (USPs) representing one of the more attractive target classes within the area. Many USPs have been linked to critical axes for therapeutic intervention, and the finding that USP28 is required for c-Myc stability suggests that USP28 inhibition may represent a novel approach to targeting this so far undruggable oncogene. Here, we describe the discovery of the first reported inhibitors of USP28, which we demonstrate are able to bind to and inhibit USP28, and while displaying a dual activity against the closest homologue USP25, these inhibitors show a high degree of selectivity over other deubiquitinases (DUBs). The utility of these compounds as valuable probes to investigate and further explore cellular DUB biology is highlighted by the demonstration of target engagement against both USP25 and USP28 in cells. Furthermore, we demonstrate that these inhibitors are able to elicit modulation of both the total levels and the half-life of the c-Myc oncoprotein in cells and also induce apoptosis and loss of cell viability in a range of cancer cell lines. We however observed a narrow therapeutic index compared to a panel of tissue-matched normal cell lines. Thus, it is hoped that these probes and data presented herein will further advance our understanding of the biology and tractability of DUBs as potential future therapeutic targets.
中文翻译:

USP25 / 28去泛素化酶亚家族的双重抑制剂的鉴定和表征
普遍认为泛素蛋白酶体系统是未来药物开发的一个新的重要领域,泛素特异性蛋白酶(USPs)代表了该区域内更具吸引力的目标类别之一。许多USP已与治疗干预的关键轴相关联,发现c-Myc稳定性需要USP28的发现表明,USP28抑制作用可能是靶向这种迄今无法耐受的癌基因的新方法。在这里,我们描述了第一个报道的USP28抑制剂的发现,我们证明了它们能够结合并抑制USP28,并且在针对最接近的同源USP25表现出双重活性的同时,这些抑制剂显示出比其他去泛素酶高的选择性( DUBs)。通过针对细胞中USP25和USP28的靶标结合,突出了这些化合物作为研究和进一步探索细胞DUB生物学的有价值的探针的实用性。此外,我们证明这些抑制剂能够引起细胞中c-Myc癌蛋白的总水平和半衰期的调节,并且还诱导一系列癌细胞系中的凋亡和细胞活力丧失。然而,与一组组织匹配的正常细胞系相比,我们观察到了较窄的治疗指数。因此,希望本文提出的这些探针和数据将进一步提高我们对作为潜在的未来治疗靶标的DUBs的生物学和易处理性的理解。我们证明这些抑制剂能够引起细胞中c-Myc癌蛋白的总水平和半衰期的调节,并且还诱导一系列癌细胞系中的细胞凋亡和细胞活力的丧失。然而,与一组组织匹配的正常细胞系相比,我们观察到了较窄的治疗指数。因此,希望本文提出的这些探针和数据将进一步提高我们对作为潜在的未来治疗靶标的DUBs的生物学和易处理性的理解。我们证明这些抑制剂能够引起细胞中c-Myc癌蛋白的总水平和半衰期的调节,并且还诱导一系列癌细胞系中的细胞凋亡和细胞活力的丧失。然而,与一组组织匹配的正常细胞系相比,我们观察到了较窄的治疗指数。因此,希望本文提出的这些探针和数据将进一步提高我们对作为潜在的未来治疗靶标的DUBs的生物学和易处理性的理解。
更新日期:2017-11-28
中文翻译:

USP25 / 28去泛素化酶亚家族的双重抑制剂的鉴定和表征
普遍认为泛素蛋白酶体系统是未来药物开发的一个新的重要领域,泛素特异性蛋白酶(USPs)代表了该区域内更具吸引力的目标类别之一。许多USP已与治疗干预的关键轴相关联,发现c-Myc稳定性需要USP28的发现表明,USP28抑制作用可能是靶向这种迄今无法耐受的癌基因的新方法。在这里,我们描述了第一个报道的USP28抑制剂的发现,我们证明了它们能够结合并抑制USP28,并且在针对最接近的同源USP25表现出双重活性的同时,这些抑制剂显示出比其他去泛素酶高的选择性( DUBs)。通过针对细胞中USP25和USP28的靶标结合,突出了这些化合物作为研究和进一步探索细胞DUB生物学的有价值的探针的实用性。此外,我们证明这些抑制剂能够引起细胞中c-Myc癌蛋白的总水平和半衰期的调节,并且还诱导一系列癌细胞系中的凋亡和细胞活力丧失。然而,与一组组织匹配的正常细胞系相比,我们观察到了较窄的治疗指数。因此,希望本文提出的这些探针和数据将进一步提高我们对作为潜在的未来治疗靶标的DUBs的生物学和易处理性的理解。我们证明这些抑制剂能够引起细胞中c-Myc癌蛋白的总水平和半衰期的调节,并且还诱导一系列癌细胞系中的细胞凋亡和细胞活力的丧失。然而,与一组组织匹配的正常细胞系相比,我们观察到了较窄的治疗指数。因此,希望本文提出的这些探针和数据将进一步提高我们对作为潜在的未来治疗靶标的DUBs的生物学和易处理性的理解。我们证明这些抑制剂能够引起细胞中c-Myc癌蛋白的总水平和半衰期的调节,并且还诱导一系列癌细胞系中的细胞凋亡和细胞活力的丧失。然而,与一组组织匹配的正常细胞系相比,我们观察到了较窄的治疗指数。因此,希望本文提出的这些探针和数据将进一步提高我们对作为潜在的未来治疗靶标的DUBs的生物学和易处理性的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号