当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(I)‐Catalyzed Arylation/Dehydroxylation of tert‐Propargylic Alcohols Leading to Tetrasubstituted Allenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-05 , DOI: 10.1002/adsc.201701263 Na Liu 1 , Yanle Zhi 1 , Jian Yao 1 , Junhao Xing 1 , Tao Lu 1 , Xiaowei Dou 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-05 , DOI: 10.1002/adsc.201701263 Na Liu 1 , Yanle Zhi 1 , Jian Yao 1 , Junhao Xing 1 , Tao Lu 1 , Xiaowei Dou 1
Affiliation
|
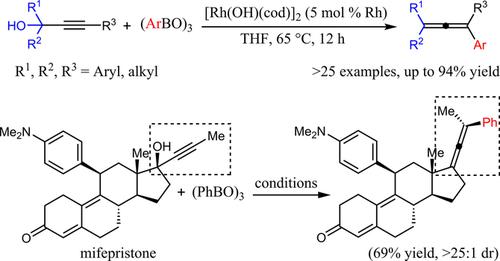
Diverse tetrasubstituted allenes are obtained selectively by the reaction of tert‐propargylic alcohols and arylboroxines under rhodium catalysis. The reaction is assumed to proceed through an arylation/dehydroxylation process, which involves β‐hydroxide elimination of a β‐hydroxy alkenyl‐rhodium intermediate that is generated by regioselective arylrhodation of the tert‐propargylic alcohol. In addition, when enantioenriched propargylic alcohol was used to prepare optically active allene, high efficiency of central‐to‐axial chirality transfer was observed. The application of current method to structural modification of pharmaceutical drugs was also showcased by a highly diastereoselective transformation of mifepristone.
中文翻译:

铑(I)催化叔丙醇的芳基化/脱羟基化,导致四取代的丙二烯
叔炔丙基醇与芳基硼氧烷在铑催化下的反应选择性地获得了不同的四取代烯。假定该反应是通过芳基化/去羟基化过程进行的,该过程涉及通过β-羟基消除叔炔丙醇的区域选择性芳基铑化反应所产生的β-羟基烯基-铑中间体。此外,当使用富含对映体的炔丙醇制备旋光性丙二烯时,观察到了高效率的中心-轴向手性转移。米非司酮的高度非对映选择性转化也展示了当前方法在药物结构修饰中的应用。
更新日期:2017-12-05
中文翻译:

铑(I)催化叔丙醇的芳基化/脱羟基化,导致四取代的丙二烯
叔炔丙基醇与芳基硼氧烷在铑催化下的反应选择性地获得了不同的四取代烯。假定该反应是通过芳基化/去羟基化过程进行的,该过程涉及通过β-羟基消除叔炔丙醇的区域选择性芳基铑化反应所产生的β-羟基烯基-铑中间体。此外,当使用富含对映体的炔丙醇制备旋光性丙二烯时,观察到了高效率的中心-轴向手性转移。米非司酮的高度非对映选择性转化也展示了当前方法在药物结构修饰中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号