当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Nitroaldol Reaction Associated with Deuterium‐Labeling
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-05 , DOI: 10.1002/adsc.201701224 Tsuyoshi Yamada 1 , Marina Kuwata 1 , Ryoya Takakura 1 , Yasunari Monguchi 1 , Hironao Sajiki 1 , Yoshinari Sawama 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-05 , DOI: 10.1002/adsc.201701224 Tsuyoshi Yamada 1 , Marina Kuwata 1 , Ryoya Takakura 1 , Yasunari Monguchi 1 , Hironao Sajiki 1 , Yoshinari Sawama 1
Affiliation
|
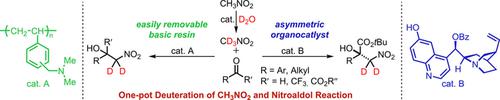
A deuterium‐labeling reaction of nitroalkanes in deuterium oxide and the subsequent nitroaldol reaction have been accomplished under basic and organocatalytic conditions to provide the deuterium‐labeled β‐nitroalcohols in high yields and high deuterium contents. β‐Deuterated β‐nitroalcohols could be smoothly obtained from the reaction of nitroalkanes and various electrophiles using the easily‐removal basic resin WA30. Furthermore, the asymmetric nitroaldol reaction using nitromethane and α‐keto esters as electrophiles in the presence of a quinine‐derived organocatalyst in deuterium oxide could provide the desired β‐deuterated nitroalcohol derivatives with high enantioselectivities.
中文翻译:

氘标记相关的有机催化硝基醛醇反应
在碱性和有机催化条件下,已经完成了氧化氘中的硝基链烷烃的氘标记反应和随后的硝基醛反应,从而以高收率和高氘含量提供了氘标记的β-硝基醇。使用易去除的碱性树脂WA30,可通过硝基烷与各种亲电试剂的反应平稳地获得β氘代的β硝基醇。此外,在奎宁衍生的有机催化剂存在下,使用硝基甲烷和α-酮酸酯作为亲电试剂的不对称硝基醛醇反应可提供所需的具有高对映选择性的β-氘代硝基醇衍生物。
更新日期:2017-12-05
中文翻译:

氘标记相关的有机催化硝基醛醇反应
在碱性和有机催化条件下,已经完成了氧化氘中的硝基链烷烃的氘标记反应和随后的硝基醛反应,从而以高收率和高氘含量提供了氘标记的β-硝基醇。使用易去除的碱性树脂WA30,可通过硝基烷与各种亲电试剂的反应平稳地获得β氘代的β硝基醇。此外,在奎宁衍生的有机催化剂存在下,使用硝基甲烷和α-酮酸酯作为亲电试剂的不对称硝基醛醇反应可提供所需的具有高对映选择性的β-氘代硝基醇衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号