Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hyaluronic Acid Layered Chimeric Nanoparticles: Targeting MAPK-PI3K Signaling Hub in Colon Cancer Cells
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acsomega.7b01315 Sandeep Palvai 1 , Meenu Mahesh Kuman 1 , Poulomi Sengupta 2 , Sudipta Basu 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acsomega.7b01315 Sandeep Palvai 1 , Meenu Mahesh Kuman 1 , Poulomi Sengupta 2 , Sudipta Basu 1
Affiliation
|
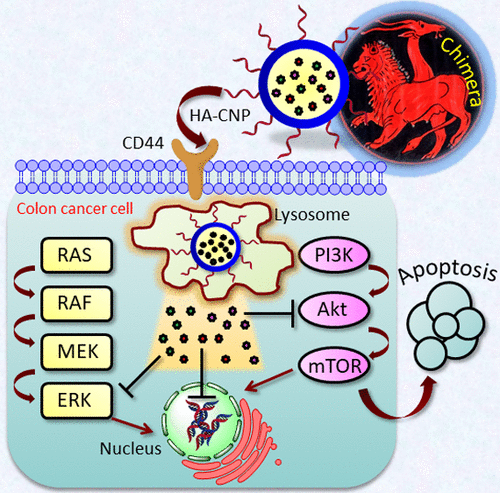
Colon cancer has emerged as one of the most devastating diseases in the whole world. Mitogen-activated protein kinase (MAPK)-phosphatidylinsitol-3-kinase (PI3K) signaling hub has gained lots of attention due to its deregulation in colon cancer cells. However, selective targeting of oncogenic MAPK-PI3K hub in colon cancer has remained highly challenging, hence it has mostly been unexplored. To address this, we have engineered a hyaluronic acid layered lipid-based chimeric nanoparticle (HA-CNP) consisting of AZD6244 (MAPK inhibitor), PI103 (PI3K inhibitor), and cisplatin (DNA impairing drug) ratiometrically in a single particle. Electron microscopy (field emission scanning electron microscopy and atomic force microscopy) and dynamic light scattering were utilized to characterize the size, shape, morphology, and surface charge of the HA-CNPs. Fluorescent confocal laser scanning microscopy and flow cytometry analysis confirmed that HA-CNPs were taken up by HCT-116 colon cancer cells by merging of clathrin and CD44 receptor-mediated endocytosis along with macropinocytosis to home into acidic organelles (lysosomes) within 1 h. A gel electrophoresis study evidently established that HA-CNPs simultaneously inhibited MAPK-PI3K signaling hub with DNA damage in HCT-116 cells. These HA-CNPs stalled the cell cycle into G0/G1 phase, leading to induction of apoptosis (early and late) in colon cancer cells. Finally, these HA-CNPs exerted remarkable cytotoxicity in HCT-116 colon cancer cells at 24 h compared to that of the free triple drug cocktail as well as HA-coated dual drug-loaded nanoparticles without showing any cell death in healthy L929 fibroblast cells. These HA-coated CNPs have potential to be translated into clinics as a novel platform to perturb various oncogenic signaling hubs concomitantly toward next-generation targeted colon cancer therapy.
中文翻译:

透明质酸层状嵌合纳米颗粒:靶向结肠癌细胞中的 MAPK-PI3K 信号中枢
结肠癌已成为全世界最具破坏性的疾病之一。丝裂原激活蛋白激酶 (MAPK)-磷脂酰肌醇-3-激酶 (PI3K) 信号中枢因其在结肠癌细胞中的失调而受到广泛关注。然而,选择性靶向结肠癌中的致癌 MAPK-PI3K 中枢仍然极具挑战性,因此大多尚未被探索。为了解决这个问题,我们设计了一种透明质酸层状脂质嵌合纳米颗粒(HA-CNP),其在单个颗粒中按比例由 AZD6244(MAPK 抑制剂)、PI103(PI3K 抑制剂)和顺铂(DNA 损伤药物)组成。利用电子显微镜(场发射扫描电子显微镜和原子力显微镜)和动态光散射来表征HA-CNP的尺寸、形状、形态和表面电荷。荧光共焦激光扫描显微镜和流式细胞仪分析证实,HA-CNP 通过网格蛋白和 CD44 受体介导的内吞作用以及巨胞饮作用的融合而被 HCT-116 结肠癌细胞摄取,并在 1 小时内归入酸性细胞器(溶酶体)。凝胶电泳研究明显证实,HA-CNP 在 HCT-116 细胞中同时抑制 MAPK-PI3K 信号中枢和 DNA 损伤。这些 HA-CNP 使细胞周期停滞在 G0/G1 期,从而诱导结肠癌细胞凋亡(早期和晚期)。最后,与游离三联药物混合物以及 HA 包被的双载药纳米颗粒相比,这些 HA-CNP 在 24 小时内对 HCT-116 结肠癌细胞表现出显着的细胞毒性,而在健康的 L929 成纤维细胞中未显示任何细胞死亡。这些 HA 包被的 CNP 有可能作为一种新的平台转化为临床,以扰乱各种致癌信号中枢,同时实现下一代靶向结肠癌治疗。
更新日期:2017-11-14
中文翻译:

透明质酸层状嵌合纳米颗粒:靶向结肠癌细胞中的 MAPK-PI3K 信号中枢
结肠癌已成为全世界最具破坏性的疾病之一。丝裂原激活蛋白激酶 (MAPK)-磷脂酰肌醇-3-激酶 (PI3K) 信号中枢因其在结肠癌细胞中的失调而受到广泛关注。然而,选择性靶向结肠癌中的致癌 MAPK-PI3K 中枢仍然极具挑战性,因此大多尚未被探索。为了解决这个问题,我们设计了一种透明质酸层状脂质嵌合纳米颗粒(HA-CNP),其在单个颗粒中按比例由 AZD6244(MAPK 抑制剂)、PI103(PI3K 抑制剂)和顺铂(DNA 损伤药物)组成。利用电子显微镜(场发射扫描电子显微镜和原子力显微镜)和动态光散射来表征HA-CNP的尺寸、形状、形态和表面电荷。荧光共焦激光扫描显微镜和流式细胞仪分析证实,HA-CNP 通过网格蛋白和 CD44 受体介导的内吞作用以及巨胞饮作用的融合而被 HCT-116 结肠癌细胞摄取,并在 1 小时内归入酸性细胞器(溶酶体)。凝胶电泳研究明显证实,HA-CNP 在 HCT-116 细胞中同时抑制 MAPK-PI3K 信号中枢和 DNA 损伤。这些 HA-CNP 使细胞周期停滞在 G0/G1 期,从而诱导结肠癌细胞凋亡(早期和晚期)。最后,与游离三联药物混合物以及 HA 包被的双载药纳米颗粒相比,这些 HA-CNP 在 24 小时内对 HCT-116 结肠癌细胞表现出显着的细胞毒性,而在健康的 L929 成纤维细胞中未显示任何细胞死亡。这些 HA 包被的 CNP 有可能作为一种新的平台转化为临床,以扰乱各种致癌信号中枢,同时实现下一代靶向结肠癌治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号