当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capturing the Elusive Water Trimer from the Stepwise Growth of Water on the Surface of the Polycyclic Aromatic Hydrocarbon Acenaphthene
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.jpclett.7b02695 Amanda L. Steber 1, 2, 3, 4 , Cristóbal Pérez 1, 2, 3, 4 , Berhane Temelso 5 , George C. Shields 5 , Anouk M. Rijs 6 , Brooks H. Pate 7 , Zbigniew Kisiel 8 , Melanie Schnell 1, 2, 3, 4
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.jpclett.7b02695 Amanda L. Steber 1, 2, 3, 4 , Cristóbal Pérez 1, 2, 3, 4 , Berhane Temelso 5 , George C. Shields 5 , Anouk M. Rijs 6 , Brooks H. Pate 7 , Zbigniew Kisiel 8 , Melanie Schnell 1, 2, 3, 4
Affiliation
|
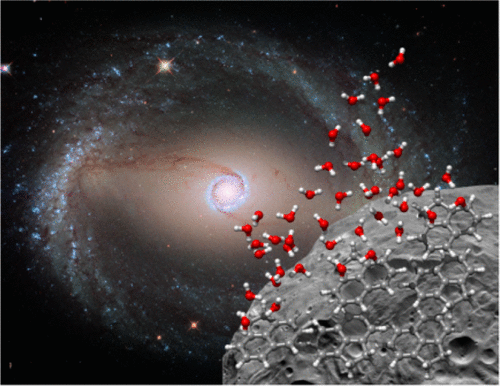
Polycyclic aromatic hydrocarbons (PAHs) are key players in reaction chemistry. While it is postulated that they serve as a basis for ice grains, there has been no direct detection of PAHs in astronomical environments. We aim to investigate the hydration of PAHs to set a foundation for the future exploration of potential ice formation pathways. We report results from chirped pulse Fourier transform microwave spectroscopy and quantum-chemical calculations for the PAH acenaphthene and acenaphthene complexed with up to four water molecules. The acenaphthene–(H2O)3 complex is of particular interest as the elusive cyclic water trimer was observed. It appears in a slightly distorted configuration when compared with the pure water trimer. This is explained by hydrogen-bond net cooperativity effects. Binding energies for the complexes are presented. Our results provide insight into the onset of complex aggregation that could be occurring in extraterrestrial environments as part of ice grain formation.
中文翻译:

从多环芳烃Ac烯表面上水的逐步生长中捕获难以捉摸的水三聚体
多环芳烃(PAH)是反应化学中的关键角色。尽管假定它们是冰粒的基础,但在天文环境中还没有直接检测到PAHs。我们旨在研究PAHs的水合作用,为将来探索潜在的冰形成途径奠定基础。我们报告from脉冲傅里叶变换微波光谱和PAH ena和最多与四个水分子配合的quantum的量子化学计算的结果。ena-(H 2 O)3由于观察到难以捉摸的循环水三聚体,因此特别关注该配合物。与纯水三聚体相比,它的外观有些变形。这可以通过氢键的净协同作用来解释。给出了配合物的结合能。我们的结果提供了对复杂聚集的开始的见解,该复杂聚集可能是在外星环境中作为冰粒形成的一部分而发生的。
更新日期:2017-11-14
中文翻译:

从多环芳烃Ac烯表面上水的逐步生长中捕获难以捉摸的水三聚体
多环芳烃(PAH)是反应化学中的关键角色。尽管假定它们是冰粒的基础,但在天文环境中还没有直接检测到PAHs。我们旨在研究PAHs的水合作用,为将来探索潜在的冰形成途径奠定基础。我们报告from脉冲傅里叶变换微波光谱和PAH ena和最多与四个水分子配合的quantum的量子化学计算的结果。ena-(H 2 O)3由于观察到难以捉摸的循环水三聚体,因此特别关注该配合物。与纯水三聚体相比,它的外观有些变形。这可以通过氢键的净协同作用来解释。给出了配合物的结合能。我们的结果提供了对复杂聚集的开始的见解,该复杂聚集可能是在外星环境中作为冰粒形成的一部分而发生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号