Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionic Conductivity Measurements—A Powerful Tool for Monitoring Polyol Reduction Reactions
Langmuir ( IF 3.7 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.langmuir.7b03444 Hany A. El-Sayed , Veronika M. Burger , Melanie Miller , Klaus Wagenbauer 1 , Manuel Wagenhofer , Hubert A. Gasteiger
Langmuir ( IF 3.7 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.langmuir.7b03444 Hany A. El-Sayed , Veronika M. Burger , Melanie Miller , Klaus Wagenbauer 1 , Manuel Wagenhofer , Hubert A. Gasteiger
Affiliation
|
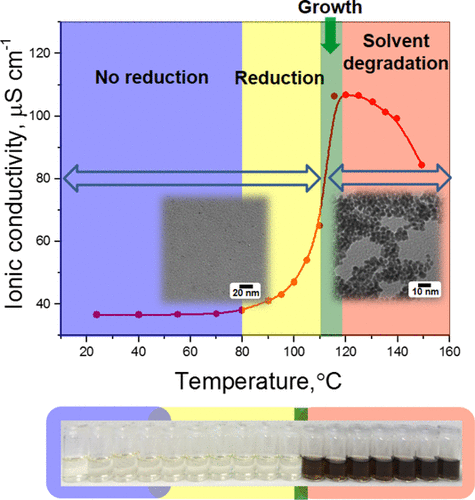
The reduction of metal precursors during the polyol synthesis of metal nanoparticles was monitored by ex situ ionic conductivity measurements. Using commonly used platinum precursors (K2PtCl6, H2PtCl6, and K2PtCl4) as well as iridium and ruthenium precursors (IrCl3 and RuCl3), we demonstrate that their reduction in ethylene glycol at elevated temperatures is accompanied by a predictable change in ionic conductivity, enabling a precise quantification of the onset temperature for their reduction. This method also allows detecting the onset temperature for the further reaction of ethylene glycol with HCl produced by the reduction of chloride-containing metal precursors (at ≈120 °C). On the basis of these findings, we show that the conversion of the metal precursor to reduced metal atoms/clusters can be precisely quantified, if the reaction occurs below 120 °C, which also enables a distinction between the stages of metal particle nucleation and growth. The latter is demonstrated by the reduction of H2PtCl6 in ethylene glycol, comparing ionic conductivity measurements with transmission electron microscopy analysis. In summary, ionic conductivity measurements are a simple and straightforward tool to quantify the reduction kinetics of commonly used metal precursors in the polyol synthesis.
中文翻译:

离子电导率测量—监测多元醇还原反应的强大工具
通过异位离子电导率测量监测在金属纳米粒子的多元醇合成期间金属前体的还原。使用常用的铂前体(K 2 PtCl 6,H 2 PtCl 6和K 2 PtCl 4)以及铱和钌前体(IrCl 3和RuCl 3),我们证明了它们在高温下在乙二醇中的还原伴随着离子电导率的可预测变化,从而能够准确定量其还原的起始温度。该方法还可以检测乙二醇与通过还原含氯化物的金属前体而产生的HCl进一步反应的起始温度(约120°C)。根据这些发现,我们表明,如果反应在低于120°C的温度下发生,则可以精确地量化金属前体向还原的金属原子/团簇的转化,这也可以区分金属颗粒成核和生长的阶段。后者通过还原H 2 PtCl 6来证明在乙二醇中,将离子电导率测量值与透射电子显微镜分析进行比较。总之,离子电导率测量是一种简单直接的工具,可以量化多元醇合成中常用金属前体的还原动力学。
更新日期:2017-11-14
中文翻译:

离子电导率测量—监测多元醇还原反应的强大工具
通过异位离子电导率测量监测在金属纳米粒子的多元醇合成期间金属前体的还原。使用常用的铂前体(K 2 PtCl 6,H 2 PtCl 6和K 2 PtCl 4)以及铱和钌前体(IrCl 3和RuCl 3),我们证明了它们在高温下在乙二醇中的还原伴随着离子电导率的可预测变化,从而能够准确定量其还原的起始温度。该方法还可以检测乙二醇与通过还原含氯化物的金属前体而产生的HCl进一步反应的起始温度(约120°C)。根据这些发现,我们表明,如果反应在低于120°C的温度下发生,则可以精确地量化金属前体向还原的金属原子/团簇的转化,这也可以区分金属颗粒成核和生长的阶段。后者通过还原H 2 PtCl 6来证明在乙二醇中,将离子电导率测量值与透射电子显微镜分析进行比较。总之,离子电导率测量是一种简单直接的工具,可以量化多元醇合成中常用金属前体的还原动力学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号