当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenation of γ-Butyrolactone to 1,4-Butanediol over CuCo/TiO2 Bimetallic Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acscatal.7b03016 Zhiwei Huang 1, 2 , Kevin J. Barnett 1 , Joseph P. Chada 1 , Zachary J. Brentzel 1 , Zhuoran Xu 1 , James A. Dumesic 1 , George W. Huber 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acscatal.7b03016 Zhiwei Huang 1, 2 , Kevin J. Barnett 1 , Joseph P. Chada 1 , Zachary J. Brentzel 1 , Zhuoran Xu 1 , James A. Dumesic 1 , George W. Huber 1
Affiliation
|
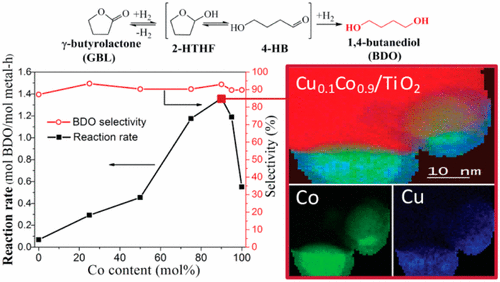
Titania-supported monometallic and bimetallic Cu–Co catalysts were prepared using (co)impregnation and studied for the hydrogenation of γ-butyrolactone (GBL) to 1,4-butanediol (BDO) at temperatures from 100 to 180 °C and a hydrogen pressure of 3.4 MPa. The highest catalytic activity occurred at a Cu:Co atomic ratio of 1:9 (Cu0.1Co0.9/TiO2), and a 95% yield of BDO was obtained. Characterization results showed mainly small nanoparticles (average size 2.6 nm) for pure Cu/TiO2, large particles (∼19.8 nm) for pure Co/TiO2, and a bimodal particle size distribution of both small (∼2.3 nm) and large (∼16.5 nm) particles for the bimetallic catalyst with a Cu:Co ratio of 1:1. The addition of ∼10 mol % Cu to Co/TiO2 increased the reducibility of the Co and resulted in the formation of core–shell CuCo bimetallic nanoparticles with a Co-rich core and Cu-rich shell. GBL hydrogenation in liquid ethanol and water produced an ester (ethyl 4-hydroxybutanoate) and a carboxylic acid (4-hydroxybutanoic acid) as the major products, respectively. GBL hydrogenation in 1,4-dioxane likely went through a 2-hydroxytetrahydrofuran (2-HTHF) intermediate. The 2-HTHF underwent facile ring-opening tautomerization to 4-hydroxybutanal (4-HB), followed by rapid hydrogenation to BDO at a reaction rate up to 700 times faster than GBL hydrogenation. The Cu0.1Co0.9/TiO2 catalyst maintained the BDO selectivity and about 80% of initial activity for GBL hydrogenation after 150 h time on stream in a continuous flow reactor.
中文翻译:

CuCo / TiO 2双金属催化剂上γ-丁内酯加氢成1,4-丁二醇
二氧化钛负载的单金属和双金属Cu-Co催化剂使用(共浸渍)制备,并研究了在100至180°C的温度和氢气压力下将γ-丁内酯(GBL)氢化为1,4-丁二醇(BDO)的过程。为3.4MPa。Cu∶Co原子比为1∶9(Cu 0.1 Co 0.9 / TiO 2)时,催化活性最高,BDO的收率为95%。表征结果显示为纯Cu /二氧化钛主要是小的纳米颗粒(平均粒径2.6纳米)2,大颗粒(~19.8纳米)为纯的Co /的TiO 2,和的双峰粒度分布既小(~2.3纳米)和大( Cu:Co比为1:1的双金属催化剂的约16.5 nm)颗粒。向Co / TiO中添加约10 mol%的Cu2提高了Co的还原性,并导致形成了具有富Co核和富Cu壳的核-壳CuCo双金属纳米颗粒。在液体乙醇和水中的GBL加氢分别生成了酯(4-羟基丁酸乙酯)和羧酸(4-羟基丁酸)作为主要产物。1,4-二恶烷中的GBL氢化反应可能通过了2-羟基四氢呋喃(2-HTHF)中间体。2-HTHF容易地开环互变异构化成4-羟基丁醛(4-HB),然后以比GBL氢化快700倍的反应速率快速氢化成BDO。Cu 0.1 Co 0.9 / TiO 2 在连续流动反应器中运行150小时后,催化剂保持BDO选择性和GBL加氢的初始活性的约80%。
更新日期:2017-11-13
中文翻译:

CuCo / TiO 2双金属催化剂上γ-丁内酯加氢成1,4-丁二醇
二氧化钛负载的单金属和双金属Cu-Co催化剂使用(共浸渍)制备,并研究了在100至180°C的温度和氢气压力下将γ-丁内酯(GBL)氢化为1,4-丁二醇(BDO)的过程。为3.4MPa。Cu∶Co原子比为1∶9(Cu 0.1 Co 0.9 / TiO 2)时,催化活性最高,BDO的收率为95%。表征结果显示为纯Cu /二氧化钛主要是小的纳米颗粒(平均粒径2.6纳米)2,大颗粒(~19.8纳米)为纯的Co /的TiO 2,和的双峰粒度分布既小(~2.3纳米)和大( Cu:Co比为1:1的双金属催化剂的约16.5 nm)颗粒。向Co / TiO中添加约10 mol%的Cu2提高了Co的还原性,并导致形成了具有富Co核和富Cu壳的核-壳CuCo双金属纳米颗粒。在液体乙醇和水中的GBL加氢分别生成了酯(4-羟基丁酸乙酯)和羧酸(4-羟基丁酸)作为主要产物。1,4-二恶烷中的GBL氢化反应可能通过了2-羟基四氢呋喃(2-HTHF)中间体。2-HTHF容易地开环互变异构化成4-羟基丁醛(4-HB),然后以比GBL氢化快700倍的反应速率快速氢化成BDO。Cu 0.1 Co 0.9 / TiO 2 在连续流动反应器中运行150小时后,催化剂保持BDO选择性和GBL加氢的初始活性的约80%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号