Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Double [3 + 2] Cycloaddition Reactions at Both C-1 and C-3 Atoms of N-Cyanomethylisoquinolinium Ylide
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acsomega.7b01391 Rong-Guo Shi 1 , Jing Sun 1 , Chao-Guo Yan 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acsomega.7b01391 Rong-Guo Shi 1 , Jing Sun 1 , Chao-Guo Yan 1
Affiliation
|
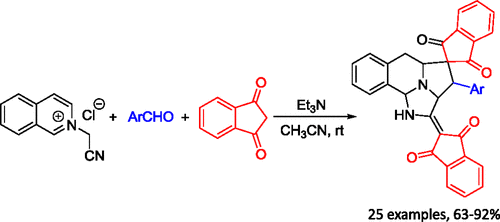
The base-promoted cycloaddition reaction of N-cyanomethylisoquinolinium chloride with 2-arylidene-1,3-indanediones in dry tetrahydrofuran resulted in the expected spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline] derivatives. However, the triethylamine-promoted three-component reaction of N-cyanomethylisoquinolinium chloride, aromatic aldehydes, and two molecules of 1,3-indanediones in acetonitrile afforded unique spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene] derivatives in satisfactory yields through tandem double [3 + 2] cycloaddition reactions.
中文翻译:

N-氰基甲基异喹啉鎓叶立德的C-1和C-3原子串联双[3 + 2]环加成反应
在干燥的四氢呋喃中,N-氰基甲基异喹啉鎓氯化物与2-亚芳基-1,3-茚满二酮的碱促进的环加成反应产生了预期的螺[indene-2,1'-吡咯并[2,1- a ]异喹啉]衍生物。然而,三乙胺促进的N-氰基甲基异喹啉鎓氯化物,芳族醛和两个分子的1,3-茚二酮在乙腈中的三组分反应提供了独特的螺[苯并[ f ]咪唑[5,1,2- cd ]吲哚嗪-通过串联双[3 + 2]环加成反应以令人满意的收率得到4,2'-茚]衍生物。
更新日期:2017-11-13
中文翻译:

N-氰基甲基异喹啉鎓叶立德的C-1和C-3原子串联双[3 + 2]环加成反应
在干燥的四氢呋喃中,N-氰基甲基异喹啉鎓氯化物与2-亚芳基-1,3-茚满二酮的碱促进的环加成反应产生了预期的螺[indene-2,1'-吡咯并[2,1- a ]异喹啉]衍生物。然而,三乙胺促进的N-氰基甲基异喹啉鎓氯化物,芳族醛和两个分子的1,3-茚二酮在乙腈中的三组分反应提供了独特的螺[苯并[ f ]咪唑[5,1,2- cd ]吲哚嗪-通过串联双[3 + 2]环加成反应以令人满意的收率得到4,2'-茚]衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号