当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Regio- and Enantioselective Nitroso Diels−Alder Reaction of 1,3-Diene-1-carbamates Catalyzed by Chiral N,N′-Dioxide/Copper(II) Complex
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-13 10:52:38 , DOI: 10.1002/adsc.201701155 Yan Lu 1 , Yuhang Zhou 1 , Jingchuan Zhang 1 , Lili Lin 1 , Xiaohua Liu 1 , Xiaoming Feng 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-13 10:52:38 , DOI: 10.1002/adsc.201701155 Yan Lu 1 , Yuhang Zhou 1 , Jingchuan Zhang 1 , Lili Lin 1 , Xiaohua Liu 1 , Xiaoming Feng 1, 2
Affiliation
|
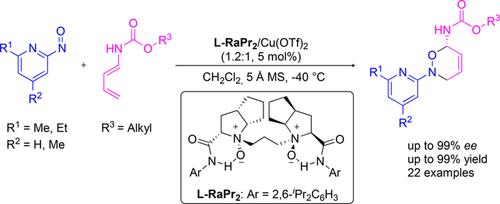
A chiral N,N′-dioxide/Copper(II) complex-catalyzed highly regio- and enantioselective nitroso Diels−Alder (NDA) reaction of 2-nitrosopyridines with 1,3-diene-1-carbamates was described. A series of 3,6-dihydro-1,2-oxazines were obtained in good to excellent yields and ee values. On the basis of the control experiments, ESI-MS analysis and the absolute configuration of the product, a possible transition state model was proposed to explain the stereocontrol.
中文翻译:

手性N,N'-二氧化物/铜(II)配合物催化1,3-二烯-1-氨基甲酸酯的高区域和对映选择性亚硝基Diels-Alder反应
描述了手性N,N'-二氧化物/铜(II)络合物催化的2-亚硝基吡啶与1,3-二烯-1-氨基甲酸酯的高度区域和对映选择性亚硝基Diels-Alder(NDA)反应。获得了一系列3,6-二氢-1,2-恶嗪,收率和ee值都非常好。在控制实验,ESI-MS分析和产品的绝对配置的基础上,提出了可能的过渡态模型来解释立体控制。
更新日期:2017-11-13
中文翻译:

手性N,N'-二氧化物/铜(II)配合物催化1,3-二烯-1-氨基甲酸酯的高区域和对映选择性亚硝基Diels-Alder反应
描述了手性N,N'-二氧化物/铜(II)络合物催化的2-亚硝基吡啶与1,3-二烯-1-氨基甲酸酯的高度区域和对映选择性亚硝基Diels-Alder(NDA)反应。获得了一系列3,6-二氢-1,2-恶嗪,收率和ee值都非常好。在控制实验,ESI-MS分析和产品的绝对配置的基础上,提出了可能的过渡态模型来解释立体控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号