当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Structure-Based Activity Study of Highly Active Unsymmetrically Substituted NHC Gold(I) Catalysts
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-13 04:40:25 , DOI: 10.1002/adsc.201701080 Poorya Zargaran 1 , Thomas Wurm 1 , David Zahner 1 , Jasmin Schießl 1 , Matthias Rudolph 1 , Frank Rominger 1 , A. Stephen K. Hashmi 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-13 04:40:25 , DOI: 10.1002/adsc.201701080 Poorya Zargaran 1 , Thomas Wurm 1 , David Zahner 1 , Jasmin Schießl 1 , Matthias Rudolph 1 , Frank Rominger 1 , A. Stephen K. Hashmi 1, 2
Affiliation
|
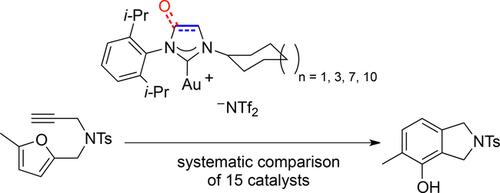
Following the modular template synthesis using isonitriles, new unsymmetrically substituted five-membered saturated N-heterocyclic carbene (NHC) and N-heterocyclic oxo-carbene (NHOC) gold(I) complexes were prepared. With these species and already reported complexes, a detailed study concerning the catalytic activities of the complex classes available by the isonitrile route was conducted. The catalytic properties of twelve different types of NHOCs, saturated and unsaturated NHC gold(I) pre-catalysts with different substituents, as well as one representative of a six-membered NHC and one N-acyclic carbene (NAC) gold(I) complex were analyzed by utilizing the phenol synthesis as a test reaction. For this reaction, the saturated NHC gold(I) complexes achieved higher conversions than the corresponding unsaturated NHCs and the NHOC systems. While unsaturated NHC complexes show higher catalytic activity during the initial phase of the conversion, due to a higher stability, higher turnover numbers (TONs) were obtained for the corresponding saturated systems. A cyclopentadecyl substituent at nitrogen turned out to be the privileged substituent for all of the unsymmetrical complexes. Furthermore, we detected that light exclusion can significantly increase the catalytic activity of NHC gold(I) complexes for phenol synthesis.
中文翻译:

高活性非对称取代NHC金(I)催化剂的基于结构的活性研究
在使用异腈进行模块化模板合成之后,制备了新的不对称取代的五元饱和N杂环卡宾(NHC)和N杂环氧代卡宾(NHOC)金(I)配合物。对于这些物种和已报道的配合物,进行了有关异腈途径可利用的配合物类别的催化活性的详细研究。十二种不同类型的NHOC,具有不同取代基的饱和和不饱和NHC金(I)预催化剂以及六元NHC和一种N-无环卡宾(NAC)金(I)配合物的代表利用苯酚合成作为测试反应进行分析。对于该反应,饱和的NHC金(I)配合物比相应的不饱和NHC和NHOC体系获得更高的转化率。尽管不饱和NHC配合物在转化的初始阶段显示出较高的催化活性,但由于具有较高的稳定性,因此相应的饱和系统获得了较高的周转率(TONs)。氮上的环十五烷基取代基被证明是所有不对称络合物的优先取代基。此外,我们检测到光排斥可以显着增加NHC金(I)配合物对苯酚合成的催化活性。
更新日期:2017-11-13
中文翻译:

高活性非对称取代NHC金(I)催化剂的基于结构的活性研究
在使用异腈进行模块化模板合成之后,制备了新的不对称取代的五元饱和N杂环卡宾(NHC)和N杂环氧代卡宾(NHOC)金(I)配合物。对于这些物种和已报道的配合物,进行了有关异腈途径可利用的配合物类别的催化活性的详细研究。十二种不同类型的NHOC,具有不同取代基的饱和和不饱和NHC金(I)预催化剂以及六元NHC和一种N-无环卡宾(NAC)金(I)配合物的代表利用苯酚合成作为测试反应进行分析。对于该反应,饱和的NHC金(I)配合物比相应的不饱和NHC和NHOC体系获得更高的转化率。尽管不饱和NHC配合物在转化的初始阶段显示出较高的催化活性,但由于具有较高的稳定性,因此相应的饱和系统获得了较高的周转率(TONs)。氮上的环十五烷基取代基被证明是所有不对称络合物的优先取代基。此外,我们检测到光排斥可以显着增加NHC金(I)配合物对苯酚合成的催化活性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号