当前位置:
X-MOL 学术
›
JAMA Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Supreme Court Ruling in Sandoz v Amgen
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamainternmed.2017.6145 Ameet Sarpatwari 1 , Abbe R. Gluck 2 , Gregory D. Curfman 2, 3, 4
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamainternmed.2017.6145 Ameet Sarpatwari 1 , Abbe R. Gluck 2 , Gregory D. Curfman 2, 3, 4
Affiliation
|
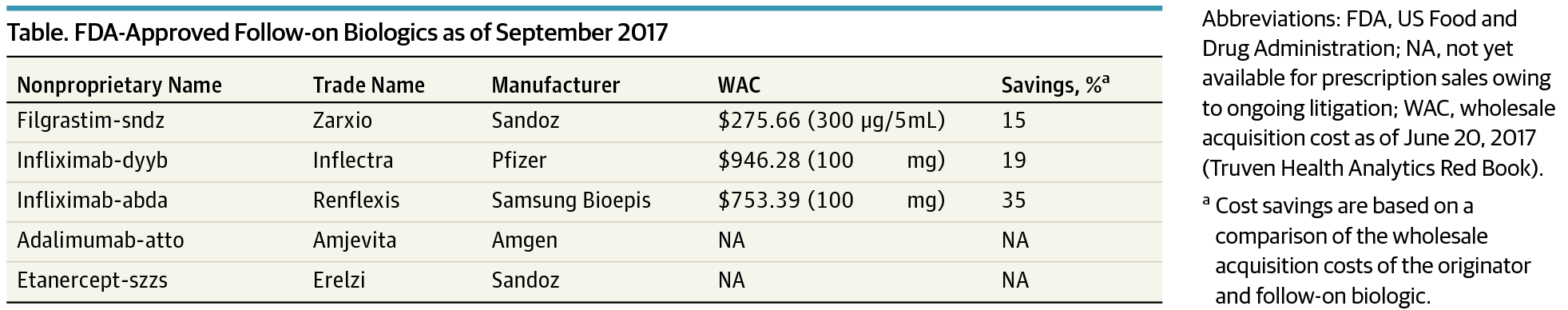
In June 2017, the Supreme Court issued its first decision concerning follow-on biologics—lower-cost versions of innovator biologics—ruling 9-0 in Sandoz v Amgen.1 The decision ruled that follow-on biologic companies are not required to share their licensing application with the innovator company and that a required 180day notice to the innovator company before commercial marketing could be given before US Food and Drug Administration (FDA) approval of their product. This handed the nascent follow-on biologics industry a substantial, albeit incomplete victory, which may help reduce prices and improve patient access to biologic therapies.
中文翻译:

最高法院在 Sandoz 诉 Amgen 案中的裁决
2017 年 6 月,最高法院在 Sandoz 诉 Amgen 案中以 9-0 的比分裁定了后续生物制剂(创新生物制剂的低成本版本)的第一个裁决。向创新公司提出许可申请,并且在美国食品和药物管理局 (FDA) 批准其产品之前,必须提前 180 天通知创新公司,然后才能进行商业营销。这为新生的后续生物制品行业带来了实质性的、尽管不完全的胜利,这可能有助于降低价格并改善患者获得生物疗法的机会。
更新日期:2018-01-01
中文翻译:

最高法院在 Sandoz 诉 Amgen 案中的裁决
2017 年 6 月,最高法院在 Sandoz 诉 Amgen 案中以 9-0 的比分裁定了后续生物制剂(创新生物制剂的低成本版本)的第一个裁决。向创新公司提出许可申请,并且在美国食品和药物管理局 (FDA) 批准其产品之前,必须提前 180 天通知创新公司,然后才能进行商业营销。这为新生的后续生物制品行业带来了实质性的、尽管不完全的胜利,这可能有助于降低价格并改善患者获得生物疗法的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号