当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclic ureas (DMI, DMPU) as efficient, sustainable ligands in iron-catalyzed C(sp2)–C(sp3) coupling of aryl chlorides and tosylates
Green Chemistry ( IF 9.3 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7gc02690k Elwira Bisz 1, 2, 3, 4 , Michal Szostak 1, 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7gc02690k Elwira Bisz 1, 2, 3, 4 , Michal Szostak 1, 1, 2, 3, 4
Affiliation
|
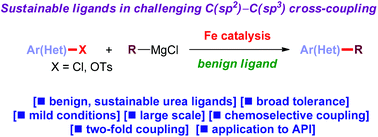
Iron-catalyzed cross-coupling has emerged as a powerful tool for sustainable catalysis. However, by far the most common ligand in iron-catalyzed cross-couplings for preparative and industrial applications is reprotoxic NMP. Herein, we report that cyclic ureas (DMI, DMPU) are efficient and sustainable alternatives to NMP in iron-catalyzed alkylations of aryl chlorides and tosylates with alkyl Grignard reagents. This environmentally benign method accomplishes traditionally challenging C(sp2)–C(sp3) cross-coupling with organometallics possessing β-hydrogens with efficiency matching or superseding NMP. The reaction is compatible with a variety of electrophilic functional handles. Applications to double and site-specific alkylations are described. The potential of this method is highlighted in the key coupling in the synthesis of a dual NK1/serotonin receptor antagonist. Considering the pivotal importance of sustainable iron-catalysis, we believe that the method will find wide application in green chemistry.
中文翻译:

环脲(DMI,DMPU)作为铁催化的芳基氯化物和甲苯磺酸盐的C(sp 2)–C(sp 3)偶联的高效,可持续的配体
铁催化的交叉偶联已经成为可持续催化的有力工具。然而,到目前为止,用于制备和工业应用的铁催化交叉偶联中最常见的配体是具有生殖毒性的NMP。在这里,我们报道在铁催化的芳基氯化物烷基化和甲苯磺酸与烷基格氏试剂的烷基化反应中,环状脲(DMI,DMPU)是NMP的有效和可持续替代品。这种对环境无害的方法可以完成传统上具有挑战性的C(sp 2)–C(sp 3)与具有β氢的有机金属进行交叉偶联,效率匹配或取代NMP。该反应与各种亲电子功能手柄兼容。描述了对双重和位点特异性烷基化的应用。双重NK1 / 5-羟色胺受体拮抗剂合成中的关键偶联突出了该方法的潜力。考虑到可持续铁催化的关键重要性,我们认为该方法将在绿色化学中得到广泛应用。
更新日期:2017-11-13
中文翻译:

环脲(DMI,DMPU)作为铁催化的芳基氯化物和甲苯磺酸盐的C(sp 2)–C(sp 3)偶联的高效,可持续的配体
铁催化的交叉偶联已经成为可持续催化的有力工具。然而,到目前为止,用于制备和工业应用的铁催化交叉偶联中最常见的配体是具有生殖毒性的NMP。在这里,我们报道在铁催化的芳基氯化物烷基化和甲苯磺酸与烷基格氏试剂的烷基化反应中,环状脲(DMI,DMPU)是NMP的有效和可持续替代品。这种对环境无害的方法可以完成传统上具有挑战性的C(sp 2)–C(sp 3)与具有β氢的有机金属进行交叉偶联,效率匹配或取代NMP。该反应与各种亲电子功能手柄兼容。描述了对双重和位点特异性烷基化的应用。双重NK1 / 5-羟色胺受体拮抗剂合成中的关键偶联突出了该方法的潜力。考虑到可持续铁催化的关键重要性,我们认为该方法将在绿色化学中得到广泛应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号