当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Depolymerization of poly(bisphenol A carbonate) under mild conditions by solvent-free alcoholysis catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene as a recyclable organocatalyst: a route to chemical recycling of waste polycarbonate
Green Chemistry ( IF 9.3 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7gc02063e Eugenio Quaranta 1, 2, 3, 4, 5 , Damiano Sgherza 1, 2, 3, 4, 5 , Giuseppe Tartaro 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7gc02063e Eugenio Quaranta 1, 2, 3, 4, 5 , Damiano Sgherza 1, 2, 3, 4, 5 , Giuseppe Tartaro 1, 2, 3, 4, 5
Affiliation
|
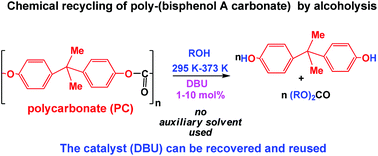
DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) is an active catalyst of poly(bisphenol A carbonate) (PC) alcoholysis and promotes selectively and quantitatively the solvent-free (no auxiliary solvent used) depolymerization of the polymeric material to bisphenol A (BPA) and the corresponding organic carbonate (RO)2CO under not severe conditions (295 K–373 K). The process is relevant to the chemical recycling and valorization of the waste polymer. Using methanol as the reference alcohol, the influence of a few experimental parameters (temperature, MeOH/PC ratio, DBU load and concentration) on the conversion time and product (BPA; dimethyl carbonate (DMC)) yield has been investigated. The catalytic activity of DBU has been compared with that of other organocatalysts such as DABCO (1,4-diazabicyclo[2.2.2]octane) and DMAP (4-(dimethylamino)pyridine), which were found to be less active than the amidine base. The study has been extended to ethanol and EtOH/MeOH mixtures. The ethanolysis reaction afforded BPA and diethyl carbonate (DEC) selectively with high yield, but proceeded more slowly than methanolysis. In MeOH/EtOH solutions the depolymerization afforded, besides BPA, a mixture of DMC, DEC, and methyl ethyl carbonate (MEC): in this way, a mixed carbonate such as MEC was synthesized in one step with satisfactory yields (60%). The recyclability of the catalyst in the depolymerization process has been investigated. At the end of the reaction the catalyst was still active. It can be reused in situ by adding a more fresh polymer at the end of each catalytic run. As well, DBU can also be recovered from the reaction mixture as a DBU–BPA adduct and effectively reused in a successive run.
中文翻译:

1,8-二氮杂双环[5.4.0]十一碳-7-烯作为可循环利用的有机催化剂,在无溶剂的条件下,在无溶剂的醇解作用下,聚碳酸双酚A的解聚反应:废弃聚碳酸酯的化学回收途径
DBU(1,8-二氮杂双环[5.4.0]十一烷基-7-烯)是聚(碳酸双酚A)(PC)醇解反应的活性催化剂,可选择性和定量地促进无溶剂(无需使用辅助溶剂)的解聚聚合材料为双酚A(BPA)和相应的有机碳酸酯(RO)2在非苛刻条件下(295 K–373 K)的CO。该过程与废聚合物的化学回收和增值有关。使用甲醇作为参考醇,研究了一些实验参数(温度,MeOH / PC比,DBU负载和浓度)对转化时间和产物(BPA;碳酸二甲酯(DMC))收率的影响。已将DBU的催化活性与其他有机催化剂(如DABCO(1,4-二氮杂双环[2.2.2]辛烷)和DMAP(4-(二甲基氨基)吡啶)的活性进行了比较,发现它们的活性均低于idine。根据。该研究已扩展到乙醇和EtOH / MeOH混合物。乙醇化反应以高收率选择性地提供了BPA和碳酸二乙酯(DEC),但其进行的速度比甲醇分解的速度慢。在MeOH / EtOH溶液中进行解聚,除了BPA以外,还使用DMC,DEC和碳酸甲乙酯(MEC)的混合物:这样,一步法合成了混合的碳酸酯(例如MEC),收率令人满意(60%)。已经研究了在解聚过程中催化剂的可回收性。在反应结束时,催化剂仍然是活性的。可以重复使用在每次催化操作结束时通过添加更多新鲜的聚合物就地进行原位处理。同样,DBU也可以作为DBU-BPA加合物从反应混合物中回收,并可以有效地在后续运行中重复使用。
更新日期:2017-11-13
中文翻译:

1,8-二氮杂双环[5.4.0]十一碳-7-烯作为可循环利用的有机催化剂,在无溶剂的条件下,在无溶剂的醇解作用下,聚碳酸双酚A的解聚反应:废弃聚碳酸酯的化学回收途径
DBU(1,8-二氮杂双环[5.4.0]十一烷基-7-烯)是聚(碳酸双酚A)(PC)醇解反应的活性催化剂,可选择性和定量地促进无溶剂(无需使用辅助溶剂)的解聚聚合材料为双酚A(BPA)和相应的有机碳酸酯(RO)2在非苛刻条件下(295 K–373 K)的CO。该过程与废聚合物的化学回收和增值有关。使用甲醇作为参考醇,研究了一些实验参数(温度,MeOH / PC比,DBU负载和浓度)对转化时间和产物(BPA;碳酸二甲酯(DMC))收率的影响。已将DBU的催化活性与其他有机催化剂(如DABCO(1,4-二氮杂双环[2.2.2]辛烷)和DMAP(4-(二甲基氨基)吡啶)的活性进行了比较,发现它们的活性均低于idine。根据。该研究已扩展到乙醇和EtOH / MeOH混合物。乙醇化反应以高收率选择性地提供了BPA和碳酸二乙酯(DEC),但其进行的速度比甲醇分解的速度慢。在MeOH / EtOH溶液中进行解聚,除了BPA以外,还使用DMC,DEC和碳酸甲乙酯(MEC)的混合物:这样,一步法合成了混合的碳酸酯(例如MEC),收率令人满意(60%)。已经研究了在解聚过程中催化剂的可回收性。在反应结束时,催化剂仍然是活性的。可以重复使用在每次催化操作结束时通过添加更多新鲜的聚合物就地进行原位处理。同样,DBU也可以作为DBU-BPA加合物从反应混合物中回收,并可以有效地在后续运行中重复使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号