当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein charge transfer absorption spectra: an intrinsic probe to monitor structural and oligomeric transitions in proteins
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-11-13 , DOI: 10.1039/c7fd00194k Mohd. Ziauddin Ansari 1, 2, 3, 4 , Amrendra Kumar 1, 2, 3, 4 , Dileep Ahari 1, 2, 3, 4 , Anurag Priyadarshi 1, 2, 3, 4 , Padmavathi Lolla 4, 5, 6, 7 , Rashna Bhandari 4, 5, 6, 7 , Rajaram Swaminathan 1, 2, 3, 4
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-11-13 , DOI: 10.1039/c7fd00194k Mohd. Ziauddin Ansari 1, 2, 3, 4 , Amrendra Kumar 1, 2, 3, 4 , Dileep Ahari 1, 2, 3, 4 , Anurag Priyadarshi 1, 2, 3, 4 , Padmavathi Lolla 4, 5, 6, 7 , Rashna Bhandari 4, 5, 6, 7 , Rajaram Swaminathan 1, 2, 3, 4
Affiliation
|
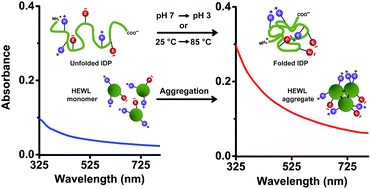
Protein Charge Transfer Spectra (ProCharTS) originate when charged amino/carboxylate groups in the side chains of Lys/Glu act as electronic charge acceptors/donors for photoinduced charge transfer either from/to the polypeptide backbone or to each other. The absorption band intensities in ProCharTS at wavelengths of 250–800 nm are dependent on the 3D spatial proximity of these charged functional groups across the protein. Intrinsically disordered proteins (IDPs) are an important class of proteins involved in signalling and regulatory functions in the eukaryotic cell. IDPs are rich in charged amino acids, but lack structure-promoting intrinsic spectral probes like Tyr or Trp in their sequences, making their structural characterisation difficult. Here, we exploit the richness of charged amino acid populations among IDPs (like the PEST fragment of human c-Myc, its mutant and dehydrin from maize) to sense structural transitions in IDPs using ProCharTS absorption spectra. Conformational changes induced in the protein by altering the pH and temperature of the aqueous medium were monitored by ProCharTS and confirmed by CD spectra. Further, the utility of ProCharTS to detect protein aggregation was examined using Hen Egg-White Lysozyme (HEWL) protein. The results revealed that in the presence of Trp/Tyr, ProCharTS absorbance was substantially reduced, specifically at wavelengths where the absorption by Trp or Tyr was near its maximum. Significant changes in the ProCharTS spectra were observed with changing pH in the range of 3–11, which correlated with changes in the secondary structure of the PEST fragment. Importantly, the absorbance at 280 nm, which is often employed as a measure of protein concentration, was profoundly altered by changes in ProCharTS intensity in response to changing the pH in dehydrin. The ProCharTS intensity was sensitive to temperature-induced changes in the secondary structures of the PEST fragments between 25–85 °C. The presence of 0.25 M NaCl or KCl in the medium also altered the ProCharTS spectrum. Finally, an increase in ProCharTS absorbance with time in HEWL at pH 2 directly correlated with the growth of HEWL aggregates and amyloid fibrils, as confirmed by the increasing thioflavin T fluorescence. Taken together, our work highlights the utility of ProCharTS as a label-free intrinsic probe to monitor changes in protein charge, structure and oligomeric state.
中文翻译:

蛋白质电荷转移吸收光谱:用于监测蛋白质中结构和低聚转变的内在探针
当Lys / Glu侧链中的带电荷氨基/羧酸基团充当电子受体/供体,用于光诱导的电荷从多肽主链之间或彼此之间转移时,就会产生蛋白质电荷转移谱(ProCharTS)。ProCharTS在250–800 nm波长处的吸收带强度取决于蛋白质上这些带电官能团在3D空间上的接近程度。固有紊乱蛋白(IDP)是一类重要的蛋白,参与真核细胞的信号传导和调节功能。IDP富含带电荷的氨基酸,但在序列中缺乏促进结构的固有光谱探针(例如Tyr或Trp),因此很难进行结构表征。这里,我们利用IDP之间带电氨基酸种群的丰富性(例如人c-Myc的PEST片段,其突变体和玉米中的脱水素)来利用ProCharTS吸收光谱检测IDP中的结构转变。通过ProCharTS监测通过改变水性介质的pH和温度在蛋白质中诱导的构象变化,并通过CD光谱确认。此外,使用鸡蛋清蛋白溶菌酶(HEWL)蛋白质检查了ProCharTS检测蛋白质聚集的效用。结果表明,在存在Trp / Tyr的情况下,ProCharTS吸光度显着降低,特别是在Trp或Tyr的吸光度接近其最大值的波长处。当pH值在3-11范围内变化时,ProCharTS光谱发生了显着变化,与PEST片段二级结构的变化有关。重要的是,响应脱水醇pH值的变化,ProCharTS强度的变化极大地改变了通常用于测量蛋白质浓度的280 nm处的吸光度。ProCharTS强度对温度引起的25-85°C之间的PEST片段二级结构变化敏感。培养基中0.25 M NaCl或KCl的存在也改变了ProCharTS光谱。最终,在pH 2的HEWL中,ProCharTS吸光度随时间的增加与HEWL聚集体和淀粉样原纤维的生长直接相关,这通过增加的硫代黄素T荧光得以证实。综上所述,我们的工作突出了ProCharTS作为无标记内在探针来监控蛋白质电荷变化的实用性,
更新日期:2018-04-17
中文翻译:

蛋白质电荷转移吸收光谱:用于监测蛋白质中结构和低聚转变的内在探针
当Lys / Glu侧链中的带电荷氨基/羧酸基团充当电子受体/供体,用于光诱导的电荷从多肽主链之间或彼此之间转移时,就会产生蛋白质电荷转移谱(ProCharTS)。ProCharTS在250–800 nm波长处的吸收带强度取决于蛋白质上这些带电官能团在3D空间上的接近程度。固有紊乱蛋白(IDP)是一类重要的蛋白,参与真核细胞的信号传导和调节功能。IDP富含带电荷的氨基酸,但在序列中缺乏促进结构的固有光谱探针(例如Tyr或Trp),因此很难进行结构表征。这里,我们利用IDP之间带电氨基酸种群的丰富性(例如人c-Myc的PEST片段,其突变体和玉米中的脱水素)来利用ProCharTS吸收光谱检测IDP中的结构转变。通过ProCharTS监测通过改变水性介质的pH和温度在蛋白质中诱导的构象变化,并通过CD光谱确认。此外,使用鸡蛋清蛋白溶菌酶(HEWL)蛋白质检查了ProCharTS检测蛋白质聚集的效用。结果表明,在存在Trp / Tyr的情况下,ProCharTS吸光度显着降低,特别是在Trp或Tyr的吸光度接近其最大值的波长处。当pH值在3-11范围内变化时,ProCharTS光谱发生了显着变化,与PEST片段二级结构的变化有关。重要的是,响应脱水醇pH值的变化,ProCharTS强度的变化极大地改变了通常用于测量蛋白质浓度的280 nm处的吸光度。ProCharTS强度对温度引起的25-85°C之间的PEST片段二级结构变化敏感。培养基中0.25 M NaCl或KCl的存在也改变了ProCharTS光谱。最终,在pH 2的HEWL中,ProCharTS吸光度随时间的增加与HEWL聚集体和淀粉样原纤维的生长直接相关,这通过增加的硫代黄素T荧光得以证实。综上所述,我们的工作突出了ProCharTS作为无标记内在探针来监控蛋白质电荷变化的实用性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号