当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Asymmetric Transfer Hydrogenation of α‐Substituted Acetophenones with Bifunctional Oxo‐Tethered Ruthenium(II) Catalysts
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-04 , DOI: 10.1002/adsc.201701227 Yamato Yuki 1 , Taichiro Touge 1 , Hideki Nara 1 , Kazuhiko Matsumura 1 , Mitsuhiko Fujiwhara 1 , Yoshihito Kayaki 2 , Takao Ikariya 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-04 , DOI: 10.1002/adsc.201701227 Yamato Yuki 1 , Taichiro Touge 1 , Hideki Nara 1 , Kazuhiko Matsumura 1 , Mitsuhiko Fujiwhara 1 , Yoshihito Kayaki 2 , Takao Ikariya 2
Affiliation
|
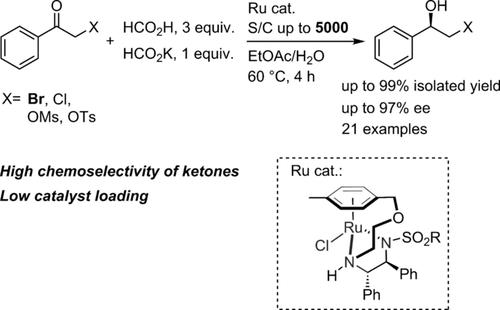
A practical method for the asymmetric transfer hydrogenation of α‐substituted ketones was developed utilizing oxo‐tethered N‐sulfonyldiamine‐ruthenium complexes. Reduction by HCO2H and HCO2K in a mixed solvent of EtOAc/H2O allowed for the selective synthesis of halohydrins from 2‐bromoacetophenone (98%) and 2‐chloroacetophenone (>99%), leading to suppressed undesired side reactions stemming from formylation under the typical reaction conditions using an azeotropic 5:2 mixture of HCO2H and Et3N. A range of functional groups, such as halogens, methoxy, nitro, dimethylamino, and ester groups, were well tolerated, highlighting the potential of this method. Nearly complete selectivity with a preferable ee was maintained even with a substrate/catalyst (S/C) ratio of 5000. This catalyst system was also effective for the asymmetric reduction of α‐sulfonated ketones without eroding the leaving group.
中文翻译:

双功能氧栓合钌(II)催化剂对α取代的苯乙酮的选择性不对称转移加氢
利用氧连接的N-磺酰基二胺-钌络合物开发了一种实用的方法,用于α-取代酮的不对称转移加氢。在EtOAc / H 2 O混合溶剂中通过HCO 2 H和HCO 2 K还原,可以从2-溴苯乙酮(98%)和2-氯苯乙酮(> 99%)选择性合成卤代醇,从而抑制了不良的副反应在典型的反应条件下,使用HCO 2 H和Et 3的5:2共沸混合物,从甲酰化反应中提取N.一系列功能基团(如卤素,甲氧基,硝基,二甲基氨基和酯基)的耐受性良好,凸显了该方法的潜力。即使底物/催化剂(S / C)为5000,也能保持接近完全的选择性和较好的ee。这种催化剂体系对于不对称地还原α-磺化酮也有效,而不会侵蚀离去基团。
更新日期:2017-12-04
中文翻译:

双功能氧栓合钌(II)催化剂对α取代的苯乙酮的选择性不对称转移加氢
利用氧连接的N-磺酰基二胺-钌络合物开发了一种实用的方法,用于α-取代酮的不对称转移加氢。在EtOAc / H 2 O混合溶剂中通过HCO 2 H和HCO 2 K还原,可以从2-溴苯乙酮(98%)和2-氯苯乙酮(> 99%)选择性合成卤代醇,从而抑制了不良的副反应在典型的反应条件下,使用HCO 2 H和Et 3的5:2共沸混合物,从甲酰化反应中提取N.一系列功能基团(如卤素,甲氧基,硝基,二甲基氨基和酯基)的耐受性良好,凸显了该方法的潜力。即使底物/催化剂(S / C)为5000,也能保持接近完全的选择性和较好的ee。这种催化剂体系对于不对称地还原α-磺化酮也有效,而不会侵蚀离去基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号