当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Observation of a Tricyclic[4.1.0.02,4]heptane During a Michael Addition-Ring Closure Reaction and a Computational Study on Its Mechanism of Formation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02218 Marco Farren-Dai 1 , John R. Thompson 1 , Anna Bernardi 2 , Cinzia Colombo 1, 2 , Andrew J. Bennet 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02218 Marco Farren-Dai 1 , John R. Thompson 1 , Anna Bernardi 2 , Cinzia Colombo 1, 2 , Andrew J. Bennet 1
Affiliation
|
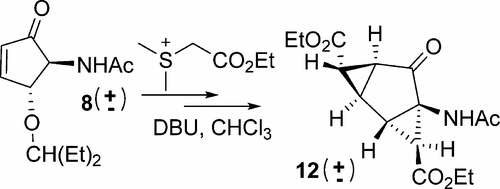
We describe the formation of a bis-cyclopropane product, a tricyclic[4.1.0.02,4]heptane, that is formed during a Johnson–Corey–Chaykovsky reaction on a cyclopentenone. Two (of four possible) bicyclic products are selectively formed by addition of a COOEt-stabilized sulfur ylide onto the Michael acceptor. The tricyclic product is formed subsequently via a retro Michael elimination of a hindered ether followed by addition of a further cyclopropyl moiety, affecting only one of the two bicyclic products initially formed. The experimental reaction outcome was rationalized using density functional theory (DFT), investigating the different Michael-addition approaches of the sulfur ylide, the transition state (TS) energies for the formation of possible zwitterionic intermediates and subsequent reactions that give rise to cyclopropanation. Selective formation of only two of the four possible products occurs due to the epimerization of unreactive intermediates from the other two pathways, as revealed by energy barrier calculations. The formation of the tricyclic product was rationalized by evaluation of energy barriers for proton abstraction required to form the intermediate undergoing the second cyclopropanation. The selectivity-guiding factors discussed for the single and double cyclopropanation of this functionalized Michael-acceptor will be useful guidelines for the synthesis of future singly and doubly cyclopropanated compounds.
中文翻译:

三环[4.1.0.0 2,4 ]庚烷在迈克尔加成环封闭反应中的观察及其形成机理的计算研究
我们描述了双环丙烷产物三环[4.1.0.0 2,4]庚烷,是在约翰逊-科里-柴科夫斯基对环戊烯酮的反应中形成的。通过将COOEt稳定的硫内鎓盐加到Michael受体上,有选择地形成两种(四种可能的)双环产物。随后通过逆向迈克尔消除受阻醚,然后加入另外的环丙基部分,形成三环产物,仅影响最初形成的两个双环产物之一。实验反应结果使用密度泛函理论(DFT)进行了合理化,研究了硫叶立德的不同迈克尔加成方法,用于形成可能的两性离子中间体的过渡态(TS)能量以及随后引起环丙烷化的反应。如能垒计算所示,由于其他两种途径中未反应的中间体的差向异构化,导致四种可能产物中只有两种的选择性形成。通过评估形成第二个环丙烷化中间体所需的质子提取能量垒,合理化了三环产物的形成。讨论的对该官能化的迈克尔受体的单环和双环丙烷化的选择性指导因素将是合成未来单和双环丙烷化化合物的有用指导。通过评估形成第二个环丙烷化中间体所需的质子提取能量垒,合理化了三环产物的形成。讨论的对该官能化的迈克尔受体的单环和双环丙烷化的选择性指导因素将是合成未来单和双环丙烷化化合物的有用指导。通过评估形成第二个环丙烷化中间体所需的质子提取能量垒,合理化了三环产物的形成。讨论的对该官能化的迈克尔受体的单环和双环丙烷化的选择性指导因素将是合成未来单和双环丙烷化化合物的有用指导。
更新日期:2017-11-11
中文翻译:

三环[4.1.0.0 2,4 ]庚烷在迈克尔加成环封闭反应中的观察及其形成机理的计算研究
我们描述了双环丙烷产物三环[4.1.0.0 2,4]庚烷,是在约翰逊-科里-柴科夫斯基对环戊烯酮的反应中形成的。通过将COOEt稳定的硫内鎓盐加到Michael受体上,有选择地形成两种(四种可能的)双环产物。随后通过逆向迈克尔消除受阻醚,然后加入另外的环丙基部分,形成三环产物,仅影响最初形成的两个双环产物之一。实验反应结果使用密度泛函理论(DFT)进行了合理化,研究了硫叶立德的不同迈克尔加成方法,用于形成可能的两性离子中间体的过渡态(TS)能量以及随后引起环丙烷化的反应。如能垒计算所示,由于其他两种途径中未反应的中间体的差向异构化,导致四种可能产物中只有两种的选择性形成。通过评估形成第二个环丙烷化中间体所需的质子提取能量垒,合理化了三环产物的形成。讨论的对该官能化的迈克尔受体的单环和双环丙烷化的选择性指导因素将是合成未来单和双环丙烷化化合物的有用指导。通过评估形成第二个环丙烷化中间体所需的质子提取能量垒,合理化了三环产物的形成。讨论的对该官能化的迈克尔受体的单环和双环丙烷化的选择性指导因素将是合成未来单和双环丙烷化化合物的有用指导。通过评估形成第二个环丙烷化中间体所需的质子提取能量垒,合理化了三环产物的形成。讨论的对该官能化的迈克尔受体的单环和双环丙烷化的选择性指导因素将是合成未来单和双环丙烷化化合物的有用指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号