当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Alkylthioetherpyrrolidine Derivatives via [3+2] Cycloaddition of α-Thioacrylates with Isocyanoacetates
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02266 Zhi-Peng Wang 1 , Zi-Rui Li 1 , Qi Wu 1 , Xiao-Jiao Peng 1 , Pan-Lin Shao 1 , Yun He 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02266 Zhi-Peng Wang 1 , Zi-Rui Li 1 , Qi Wu 1 , Xiao-Jiao Peng 1 , Pan-Lin Shao 1 , Yun He 1
Affiliation
|
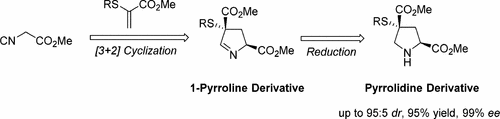
An unprecedented catalytic asymmetric method for the [3+2] cycloaddition of isocyanoacetates with α-thioacrylates/α-phthalimidoacrylates has been developed with excellent enantioselectivities. The generated pyrrolines could be readily further reduced to an array of structurally various and biologically important pyrrolidine derivatives. α-Tosyloxyacrylate with isocyanoacetates as well as tosylmethylisocyanide could be used to produce 2,4-disubstituted pyrroles.
中文翻译:

通过[3 + 2]α-硫代丙烯酸酯与异氰基乙酸酯的环加成反应,对烷硫基醚吡咯烷衍生物进行对映选择性合成
已开发出一种空前的催化不对称方法,用于异氰酸酯与α-硫代丙烯酸酯/α-邻苯二甲酰亚胺基丙烯酸酯的[3 + 2]环加成反应,具有出色的对映选择性。产生的吡咯啉可以容易地进一步还原为一系列结构上不同且生物学上重要的吡咯烷衍生物。具有异氰基乙酸酯的α-甲苯磺酰氧基丙烯酸以及甲苯磺酰基甲基异氰化物可用于生产2,4-二取代的吡咯。
更新日期:2017-11-11
中文翻译:

通过[3 + 2]α-硫代丙烯酸酯与异氰基乙酸酯的环加成反应,对烷硫基醚吡咯烷衍生物进行对映选择性合成
已开发出一种空前的催化不对称方法,用于异氰酸酯与α-硫代丙烯酸酯/α-邻苯二甲酰亚胺基丙烯酸酯的[3 + 2]环加成反应,具有出色的对映选择性。产生的吡咯啉可以容易地进一步还原为一系列结构上不同且生物学上重要的吡咯烷衍生物。具有异氰基乙酸酯的α-甲苯磺酰氧基丙烯酸以及甲苯磺酰基甲基异氰化物可用于生产2,4-二取代的吡咯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号