当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis Acid Triggered Vinylcyclopropane–Cyclopentene Rearrangement
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02351 Olga A. Ivanova 1 , Alexey O. Chagarovskiy 2, 3 , Alexey N. Shumsky 4 , Vasiliy D. Krasnobrov 1 , Irina I. Levina 4 , Igor V. Trushkov 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02351 Olga A. Ivanova 1 , Alexey O. Chagarovskiy 2, 3 , Alexey N. Shumsky 4 , Vasiliy D. Krasnobrov 1 , Irina I. Levina 4 , Igor V. Trushkov 2, 3
Affiliation
|
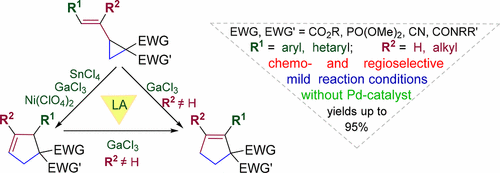
We report a mild Lewis acid induced isomerization of donor–acceptor cyclopropanes, containing an alkenyl moiety and diverse electron-withdrawing group(s) at the adjacent positions, into substituted cyclopentenes. We have found that 1,1,2-trisubstituted cyclopent-3-enes were exclusively obtained in yield of 51–99% when cyclopropanes with a 2-substituted alkenyl group as a donor underwent isomerization. For cyclopropanes bearing a trisubstituted alkenyl group either the corresponding cyclopent-3-enes or isomeric cyclopent-2-enes having two acceptor groups at the C(1) atom were formed, with the reaction selectivity being determined by the applied Lewis acid. We have shown that the reactivity of the donor–acceptor cyclopropane increases with the increase of the electron-donating character of (hetero)aromatic group attached to the alkenyl moiety. The synthetic utility of the developed methodology was also demonstrated through the synthesis of polysubstituted cyclopentane and piperidine derivatives.
中文翻译:

Lewis酸引发的乙烯基环丙烷-环戊烯重排
我们报道了一种温和的路易斯酸诱导的供体-受体环丙烷异构化为取代的环戊烯,该环含一个烯基部分和在相邻位置的多个吸电子基团。我们发现,当带有2-取代烯基作为供体的环丙烷进行异构化反应时,仅获得1,1,2-三取代的环戊-3-烯,收率为51-99%。对于带有三取代烯基的环丙烷,形成了相应的环戊-3-烯或在C(1)原子上具有两个受体基团的异构环戊-2-烯,反应选择性由所用的路易斯酸决定。我们已经表明,供体-受体环丙烷的反应性随与烯基部分连接的(杂)芳族基团的供电子特性的增加而增加。
更新日期:2017-11-11
中文翻译:

Lewis酸引发的乙烯基环丙烷-环戊烯重排
我们报道了一种温和的路易斯酸诱导的供体-受体环丙烷异构化为取代的环戊烯,该环含一个烯基部分和在相邻位置的多个吸电子基团。我们发现,当带有2-取代烯基作为供体的环丙烷进行异构化反应时,仅获得1,1,2-三取代的环戊-3-烯,收率为51-99%。对于带有三取代烯基的环丙烷,形成了相应的环戊-3-烯或在C(1)原子上具有两个受体基团的异构环戊-2-烯,反应选择性由所用的路易斯酸决定。我们已经表明,供体-受体环丙烷的反应性随与烯基部分连接的(杂)芳族基团的供电子特性的增加而增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号