当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
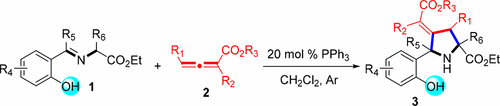
Hydroxy-Assisted Regio- and Stereoselective Synthesis of Functionalized 4-Methylenepyrrolidine Derivatives via Phosphine-Catalyzed [3 + 2] Cycloaddition of Allenoates with o-Hydroxyaryl Azomethine Ylides
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02560 Zhusheng Huang 1 , Yishu Bao 1 , Yu Zhang 1 , Fulai Yang 1 , Tao Lu 1 , Qingfa Zhou 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.joc.7b02560 Zhusheng Huang 1 , Yishu Bao 1 , Yu Zhang 1 , Fulai Yang 1 , Tao Lu 1 , Qingfa Zhou 1
Affiliation
|
In this work, we present a new strategy for the chemo-, regio-, and stereoselective synthesis of functionalized pyrrolidine derivatives via a hydroxy-assisted phosphine-catalyzed reaction of allenoates or substituted allenoates with o-hydroxyaryl azomethine ylides that offers a wide variety of 4-methylenepyrrolidine derivatives in synthetically useful yields with high stereoselctivities under mild conditions. Remarkably, it is the first example of highly regio- and stereoselective phosphine-catalyzed [3 + 2] cycloaddition of allenoates with o-hydroxyaryl azomethine ylides.
中文翻译:

通过膦催化的[3 + 2]烯丙基酸酯与邻羟基芳基甲亚胺基叶立德的羟基加成反应,羟基官能和立体选择性合成功能化的4-亚甲基吡咯烷衍生物。
在这项工作中,我们提出了一种新的策略,用于化学,区域和立体选择性合成官能化的吡咯烷衍生物,方法是通过羟基辅助膦或异戊酸酯与邻羟基芳基偶氮甲亚胺的羟基的膦化膦催化反应来实现的。 4-亚甲基吡咯烷衍生物在温和条件下具有高立体选择性的合成有用的收率。值得注意的是,这是脲基酸酯与邻羟基芳基偶氮甲亚胺基团的高区域选择性和立体选择性膦催化的[3 + 2]环加成反应的第一个实例。
更新日期:2017-11-11
中文翻译:

通过膦催化的[3 + 2]烯丙基酸酯与邻羟基芳基甲亚胺基叶立德的羟基加成反应,羟基官能和立体选择性合成功能化的4-亚甲基吡咯烷衍生物。
在这项工作中,我们提出了一种新的策略,用于化学,区域和立体选择性合成官能化的吡咯烷衍生物,方法是通过羟基辅助膦或异戊酸酯与邻羟基芳基偶氮甲亚胺的羟基的膦化膦催化反应来实现的。 4-亚甲基吡咯烷衍生物在温和条件下具有高立体选择性的合成有用的收率。值得注意的是,这是脲基酸酯与邻羟基芳基偶氮甲亚胺基团的高区域选择性和立体选择性膦催化的[3 + 2]环加成反应的第一个实例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号